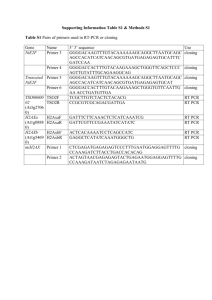
Taq purification protocol
advertisement

Taq purification protocol (source unknown) Make sure you have all chemicals. Make all (or most) of the solutions Streak a plate of TTQ #5.1 (AMP resistant) The Day Before the Prep 1. Prepare DEAE column Column removes DNA and some proteins, does not bind TAQ 2.7cm x 10cm column Mix 10X buffer (100mM Tris, 2M KCl, 0.5% Tween, 0.5% NP40) with DEAE in slurry. Do not use stir bar! Decant fines, pour column at 4°C Pre-equilibrate with 10 bed volumes of Buffer B 200mM (10mM Tris pH 7.9 at 4°C, 200mM KCl, 1mM EDTA, 1µM PMSF, 0.5% Tween-20, 0.5% NP40) 2. Biorex70 column preparation Column binds some proteins including TAQ, TAQ then eluted off 1.1cm x 15cm column Mix 10X buffer (200mM HEPES pH 7.9, 500mM KCl, 1mM EDTA, 0.5% Tween 20, 0.5% NP40) with Biorex slurry. Do not use stir bar! Decant fines, pour column at 4°C. Pre-equilibrate with 10 bed volumes of Buffer C 50mM KCl (20mM HEPES pH 7.9, 50mM KCl, 1mM EDTA, 0.5% Tween 20, 0.5% NP40) 3. Inoculate 10ml LB plus 40µg/ml Amp with one E.coli colony TTQ #5.1 (Taq plasmid driven by IPTG inducible promoter, host BL21) Day 1: 1. Finish column preparation if not yet complete 2. Save 1ml LB before inoculation (to get OD of blank) 3. Inoculate 3 x 330ml LB Amp in 2L flasks with 2ml of overnight culture. Grow on shaking incubator until OD600 = 0.25 4. Add 3ml of 20mg/ml sterile IPTG to each flask and incubate o/n [0.2g in 10ml ddH2O] Day 2: 1. Turn on 75°C waterbath 2. Check OD of cells, remove 3ml culture, spin cells down and save pellet. 3. Spin down cells in GSA Sorvall 10 min at 8000rpm, each in own centrifuge tube 4. Wash cells in 66.7ml of Buffer A (50mM Tris pH 7.9 (rt), 50mM glucose, 1mM EDTA) per tube by resuspending (vortex) and repeat spin. Pour off supernatant. 5. Resuspend in 13.3ml Buffer A with 4mg/ml lysozyme each (0.16g lysozyme in 40ml) should PMSF be present? 6. Incubate at room temperature 15 min. Add 13.3 ml Buffer B 50mM KCl (10mM Tris pH7.9 (rt), 50mM KCl, 1mM EDTA, 1mM PMSF, 0.5% Tween, 0.5% NP40) to each. 7. Transfer to glass Erlenmeyer flasks (cover with plastic wrap with holes) and incubate at 75°C for 1 hour. 8. Spin 15 min. at 8000 rpm. Keep supernatant. Save a sample for analysis (PCR cleared lysates to check)! 9. Bring solution up to 200mM KCl with 4M KCl. Check conductivity vs. DEAE wash. 10. Apply to DEAE column pre-equilibrated with 10 bed volumes of buffer (10mM Tris pH 7.9 at 4°C. 200 mM KCl, 1mM EDTA, 1mM PMSF, 0.5% Tween-20, 0.5% NP40) 11. Collect 5ml fractions – pool first peak (yellow color), save a sample for PCR analysis. Yellow pool volume approximately = input volume 12. Dilute pool to 50mM KCl with Buffer C (20mM HEPES pH 7.9, 1mM EDTA, 0.5mM PMSF, 0.5% Tween-20, 0.5% NP40, NO KCl!) Check conductivity vs. BioRex column wash, save 200µl sample for PCR. 13. Apply top BioRex70 column and set pump to run o/n. Day 3: 1. Wash column with 90ml of buffer C 50mM KCl (20mM HEPES pH 7.9, 50mM KCl, 1mM EDTA, 0.5% Tween 20, 0.5% NP40) 2. Elute with Buffer C 200mM KCl (20mM HEPES pH 7.9, 200mM KCl, 1mM EDTA, 0.5% Tween 20, 0.5% NP40). Collect 1ml fractions. 3. Assay TAQ peak by PCR using 1µl from each of first 20 fractions. 4. Pool peak fractions and dialyze vs. 100x volume of storage buffer. Change buffer twice in 24 hours – volume will decrease! 5. Titer to determine most efficient dilution. Freeze aliquots at –80°C.