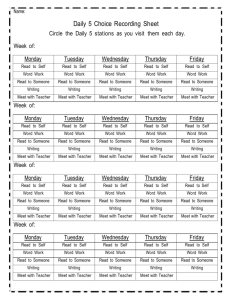
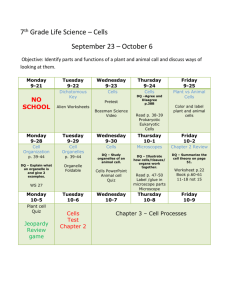
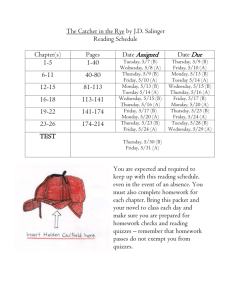
Physics 2009
advertisement

Physics Motion and Forces 1. Newton’s laws predict the motion of most objects. As a basis for understanding this concept: a. Students know how to solve problems that involve constant speed and average speed. b. Students know that when forces are balanced, no acceleration occurs; thus an object continues to move at a constant speed or stays at rest (Newton’s first law). c. Students know how to apply the law FÊ =Ê ma to solve one-dimensional motion problems that involve constant forces (Newton’s second law). d. Students know that when one object exerts a force on a second object, the second object always exerts a force of equal magnitude and in the opposite direction (Newton’s third law). e. Students know the relationship between the universal law of gravitation and the effect of gravity on an object at the surface of Earth. f. Students know applying a force to an object perpendicular to the direction of its motion causes the object to change direction but not speed (e.g., Earth’s gravitational force causes a satellite in a circular orbit to change direction but not speed). g. Students know circular motion requires the application of a constant force directed toward the center of the circle. h.* Students know Newton’s laws are not exact but provide very good approximations unless an object is moving close to the speed of light or is small enough that quantum effects are important. i.* Students know how to solve two-dimensional trajectory problems. j.* Students know how to resolve two-dimensional vectors into their components and calculate the magnitude and direction of a vector from its components. k.* Students know how to solve two-dimensional problems involving balanced forces (statics). l.* Students know how to solve problems in circular motion by using the formula for centripetal acceleration in the following form: aÊ =Êv2/r. m.* Students know how to solve problems involving the forces between two electric charges at a distance (Coulomb’s law) or the forces between two masses at a distance (universal gravitation). Conservation of Energy and Momentum 2. The laws of conservation of energy and momentum provide a way to predict and describe the movement of objects. As a basis for understanding this concept: a. Students know how to calculate kinetic energy by using the formula EÊ =Ê(1/2)mv2. b. Students know how to calculate changes in gravitational potential energy near Earth by using the formula (change in potential energy) =Ê mgh (h is the change in the elevation). c. Students know how to solve problems involving conservation of energy in simple systems, such as falling objects. d. Students know how to calculate momentum as the product mv. e. Students know momentum is a separately conserved quantity different from energy. f. Students know an unbalanced force on an object produces a change in its momentum. g. Students know how to solve problems involving elastic and inelastic collisions in one dimension by using the principles of conservation of momentum and energy. h.* Students know how to solve problems involving conservation of energy in simple systems with various sources of potential energy, such as capacitors and springs. GRADES NINE THROUGH TWELVE—PHYSICS Heat and Thermodynamics 3. Energy cannot be created or destroyed, although in many processes energy is transferred to the environment as heat. As a basis for understanding this concept: a. Students know heat flow and work are two forms of energy transfer between systems. b. Students know that the work done by a heat engine that is working in a cycle is the difference between the heat flow into the engine at high temperature and the heat flow out at a lower temperature (first law of thermodynamics) and that this is an example of the law of conservation of energy. c. Students know the internal energy of an object includes the energy of random motion of the object’s atoms and molecules, often referred to as thermal energy. The greater the temperature of the object, the greater the energy of motion of the atoms and molecules that make up the object. d. Students know that most processes tend to decrease the order of a system over time and that energy levels are eventually distributed uniformly. e. Students know that entropy is a quantity that measures the order or disorder of a system and that this quantity is larger for a more disordered system. f.* Students know the statement “Entropy tends to increase” is a law of statistical probability that governs all closed systems (second law of thermodynamics). g.* Students know how to solve problems involving heat flow, work, and efficiency in a heat engine and know that all real engines lose some heat to their surroundings. Waves 4. Waves have characteristic properties that do not depend on the type of wave. As a basis for understanding this concept: a. Students know waves carry energy from one place to another. b. Students know how to identify transverse and longitudinal waves in mechanical media, such as springs and ropes, and on the earth (seismic waves). c. Students know how to solve problems involving wavelength, frequency, and wave speed. d. Students know sound is a longitudinal wave whose speed depends on the properties of the medium in which it propagates. e. Students know radio waves, light, and X-rays are different wavelength bands in the spectrum of electromagnetic waves whose speed in a vacuum is approximately 3Ê Ê 108 m/s (186,000 miles/second). f. Students know how to identify the characteristic properties of waves: interference (beats), diffraction, refraction, Doppler effect, and polarization. Electric and Magnetic Phenomena 5. Electric and magnetic phenomena are related and have many practical applications. As a basis for understanding this concept: a. Students know how to predict the voltage or current in simple direct current (DC) electric circuits constructed from batteries, wires, resistors, and capacitors. b. Students know how to solve problems involving Ohm’s law. c. Students know any resistive element in a DC circuit dissipates energy, which heats the resistor. Students can calculate the power (rate of energy dissipation) in any resistive circuit element by using the formula Power = IR (potential difference) I (current) = I2R. d. Students know the properties of transistors and the role of transistors in electric circuits. e. Students know charged particles are sources of electric fields and are subject to the forces of the electric fields from other charges. f. Students know magnetic materials and electric currents (moving electric charges) are sources of magnetic fields and are subject to forces arising from the magnetic fields of other sources. g. Students know how to determine the direction of a magnetic field produced by a current flowing in a straight wire or in a coil. h. Students know changing magnetic fields produce electric fields, thereby inducing currents in nearby conductors. i. Students know plasmas, the fourth state of matter, contain ions or free electrons or both and conduct electricity. j.* Students know electric and magnetic fields contain energy and act as vector force fields. k.* Students know the force on a charged particle in an electric field is qE, where E is the electric field at the position of the particle and q is the charge of the particle. GRADES NINE THROUGH TWELVE—PHYSICS l.* Students know how to calculate the electric field resulting from a point charge. m.* Students know static electric fields have as their source some arrangement of electric charges. n.* Students know the magnitude of the force on a moving particle (with charge q) in a magnetic field is qvB sin(a), where a is the angle between v and B (v and B are the magnitudes of vectors v and B, respectively), and students use the right-hand rule to find the direction of this force. o.* Students know how to apply the concepts of electrical and gravitational potential energy to solve problems involving conservation of energy. Physics Syllabus Mr. Brown 2010-2011 Welcome to Physics! Physics is the oldest science, answering the “big questions” about space, energy and, time in the universe. This course is an advanced science class intended to prepare students to succeed in entry-level college science classes. Topics Outline: 1. 2. 3. 4. 5. Mechanics: Laws of Motion Energy Heat and Thermodynamics Waves Electricity and Magnetism Textbook: Conceptual Physics by Paul G. Hewitt (Prentice Hall). Each student is assigned a textbook for which (s)he is responsible. It may be kept at home. A class set of textbooks will be kept in the classroom. Supplies: Students are expected to have access to a scientific calculator at home. If you bring your personal calculator to school, you do so at your own risk. Calculators will be provided in class when required. Students are expected to bring to every class: o A 3-ring binder with section for physics o Pen or pencil Grading: Your academic grade will be calculated as follows: 40% - tests and quizzes 35% - lab work and reports 15% - homework 10% - mad minute (Journal entries and practice worksheets) Homework: Except on rare occasions, homework will be assigned every night. Late work (that is, work that is first submitted after the due date) will receive a maximum of ½ credit, except in the case of an excused absence. Absences: If you miss school, you must go to the office to have your absence excused. When your absence is excused, you must come check with Mr. Brown before class or during lunch to get assignments for the day. You will not be given the opportunity to make up work if your absence is unexcused. Tardy: Students are expected to be in their seats silently working on the mad minute when the bell rings. Students who are late will not receive credit for the mad minute. Hall Passes: Try and take care of all needs (trips to lockers, bathrooms, water fountain, office, etc.) before class. Leave one of your ten “emergency” passes at Mr. Brown’s desk if you have to take care of a need. Collect your hall pass after you return to class. Lab Safety: Safety is our number one priority in lab. Students and their parent(s) must sign a Science Lab Safety Contract before participating in any lab activities. Students who break this contract, misuse lab equipment or, fail to follow written or verbal directions will be immediately removed from the lab area of the class room, given up to two verbal warnings for misconduct and, seated at their desks for the remainder of the lab period to complete written projects that will be provided for students who fail to conduct themselves in a safe and responsible manner during lab experiments. A third warning will result in a referral in accordance with the current Y.E.S. Student Handbook. Mr. Brown’s contact info. At school: (510) 879-8877 (you can leave a message with the office) Room 304 Office Hours: Tuesday and Thursday 3:45 – 4:45, 6th period everyday By e-mail: charles.brown@ousd.k12.ca.us Classroom Norms: 1. Show respect when others are speaking by giving them your full attention and listening silently. 2. Raise your hand to comment or question. 3. Keep hands, feet, objects and, negative comments to yourself. 4. Be on time with all class materials. 5. Take care of your needs (trips to lockers, bathrooms, water fountain, office, etc.) in between classes. 6. No personal electronics (including cell phones, iPods, etc.). 7. Follow all written and verbal direction during lab to ensure your personal safety and the safety of your school community. Consequences for choosing to violate our class norms: 1. Student will receive two (2) verbal warnings in the class for behavior that disrupts the learning environment. 2. The first occurrence of a classroom disruption occurs at a repeat disruption after 2 warnings in the class. The student will be referred in writing to the Principle and the student’s parent will get a phone call from the teacher in accordance with the current Y.E.S. Student Handbook. Misconduct may also result in a call to the School Security Officer and a change of setting for the student. Student receives no credit for participation for the day. Students: I have read this classroom plan, understand it and, agree to honor our classroom policies. Signature: _____________________________________ Date: ___________________ Lesson Plans: Physics Monday 8/31/09 Tuesday 9/01/09 Wednesday 9/02/09 Thursday 9/03/09 Friday 9/04/09 Monday 9/7/09 Tuesday 9/8/09 Wednesday 9/9/09 Formative Assessment #25 Phases of the Moon Total Eclipse Video Seating Assignments How to do Journal Writing (KWL) View Video “Total Eclipse” Classroom Rules (10 minutes) Classroom Management (5 minutes Detention Rules (5 minutes) Physics Standards: Overview (10 minutes) Syllabus Labor Day—No Class Lesson 1.1-3 (Science and Creativity, Physics and Its Relation to Other Fields, Models, Theories, Laws and Principles) Read p. 1 (Introduction) closely & pp. 2-6. List and discuss terms and concepts. HW: Which of the following statements is a scientific claim Worksheet: Math in Physics: Algebra (I)-- Mathematical Review Appendices A-1 to A-4, pp. 1036-1042. For example A-4 p. 1042, show how this can can be solved alternatively by completing the square—due 9/9/09 Lesson 1.4 (Measurement and Uncertainty) Read pp. 6-8 closely & model conceptual example 1-1 p.8 and problem 1 p.16 Thursday 9/10/09 Friday 9/11/09 Formative Assessment#24: Gazing at the Moon Assign problems 2-11.pp. 16 & 17 due 9/11/09 Read at home Mathematical Review Appendices A-6 , A-7 and A-8, pp. 10431045. Draw one cycle (i.e. 0<<2) of the curves for cos () and –sin() on the same graph. How much of a cycle are these functions out of phase?—due 9/11/09 Lesson 1.5-6 (Units, standards, the SI system and Converting Units) Read pp. 8-12 closely model examples 1-2, 3 4pp.11-12 and problem 12 p.17 Assign problems 13-22 p. 17 due 9/14/09 Read at home Mathematical Review Appendices A-9 and B pp.1046-1050. What is an equivalent expression for y=ealnx? Write a brief paragraph, with an example, on the use of dimensional analysis in converting units.—due 9/14/09 Assign for home reading section 1-7 (Order of Magnitude: Rapid Estimating), pp. 12-15. Assign problems 24, 28 and 30 p. 17 HEADS UP!!! Monday 9/14/09 will be a Review and Assessment day for “Measurement” Lesson Plans: Physics Monday 9/14/09 Lesson 1.4 (Measurement and Uncertainty)—continuation Turn in homework due today. Review Appendices A-1 (Relationships, proportionality and equations), A-2 (exponents), A-3 (powers of 10), A-4 (Algebra)and A-5 (Binomial Expansion). Model examples A-1 to A-4. For example A-4 p. 1042, show how this can be solved alternatively by completing the square. Homework: access at least one (1) of the following websites and evaluate its usefulness to you for learning algebra—due 9/15/09 o http://www.algebrahelp.com/index.jsp A really good free site with useful problems and tutorials o http://www.sosmath.com/algebra/algebra.html S.O.S. Math Algebra may be a good site for problems and tutorials o Tuesday 9/15/09 Wednesday 9/16/09 http://www.algebra.com/ Another useful free site with instruction HEADS UP!!! There will be a short quiz on Wednesday. Lesson 1.4 (Measurement and Uncertainty)—continuation Discuss web-based algebra tutorials. 5-minute Quiz on appendices A-1 to A-5. Review Appendices A-6 (Plane Geometry) and A-7 (Areas and Volumes). Homework: Geometry review—problems 1-9 (even) of handout, “Lesson 8.7— Surface Area” and, 1-12 (odd) of handout, “Lesson 10.6—Volume of a Sphere”, due 9/16/09 Lesson 1.4 (Measurement and Uncertainty)—continuation Discuss web-based algebra tutorials. 5-minute Quiz on appendices A-1 to A-5. Review Appendices A-6 (Plane Geometry) and A-7 (Areas and Volumes). Homework: Geometry review—problems 1-9 (even) of handout, “Lesson 8.7— Surface Area” and, 1-12 (odd) of handout, “Lesson 10.6—Volume of a Sphere”, due 9/18/09 Bring your textbook to class on block day (Thursday) Thursday 9/17/09 Friday 9/18/09 Lesson 1.4 (Measurement and Uncertainty)--continuation Review Appendices A-8 (Trigonometric Functions and Identities) and A-9 (Logarithms). Derive trigonometric identities from the Pythagorean theorem. Draw one cycle (i.e. 0<<2) of the curves for cos () and –sin() on the same graph. How much of a cycle are these functions out of phase? What is an equivalent expression for y=e alnx ? Model problems 1,3, 13 and 15 from handout “Lesson 12.2—Problem Solving with Right Triangles”. Create a trigonometric table on a spreadsheet. Define vectors, components of vectors and conventions for adding and subtracting vectors. Homework: Geometry Review—problems 2, 4, 8, 10 and 14 of handout “Lesson 12.2— Problem Solving with Right Triangles”—due 9/21/09 Lesson 1.5-6 (Units, standards, the SI system and Converting Units) Read pp. 8-12 closely model examples 1-2, 3 4pp.11-12 and problem 12 p.17 Assign problems 13-22 p. 17 due 9/21/09 Write a brief paragraph, with an example, on the use of dimensional analysis in converting units.—due 9/21/09 Monday 9/21/09 Tuesday 9/22/09 Wednesday 9/23/09 Thursday 9/24/09 Friday 9/25/09 Assign for home reading section 1-7 (Order of Magnitude: Rapid Estimating), pp. 12-15. Assign problems 24, 28 and 30 p. 17 HEADS UP!!! Monday 9/21/09 will be a Review and Assessment day for “Measurement” Lesson 2.1-2.3 (Motion: Kinematics in One Dimension—Displacement and Velocity Read pp. 19-23. Define kinematics, displacement, average speed, average velocity and instantaneous velocity. Discuss http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html webbased objectives. Model the displacement vector on coordinate axes. Model examples 2-1 & 2-2 pp. 22 & 23 on average velocity. Model figure 2-8 at board to describe instantaneous velocity (noting similarity to slope). Do Physlet problems 1-4, in the above referenced website, on monitor with the class. Quiz: Questions 1-11, 13, p. 41, Quiz: Problems 1-5, 7, 9, 10, 11, pp. 42-43, Recommended: Buy a scientific calculator for home use before 9/23/09 Lesson 2.8 (Graphical Analysis of Linear Motion) and 2.4 (Acceleration) Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html , Practice Questions for chapter 2, and model questions 1-5, 7 , 8 , 10 & 11with class. Read PE pp. 38-40. Model fig. 2-23, p. 39 (x vs t and v vs. t) to show that v=x/t. Model example 2-16, p. 40 to show that v*t=x. Model the calculation of area under linear curves using area formulae for triangles and rectangles. Read section 2-4 (acceleration), pp. 24-26. Define average acceleration. Model examples 2-3. Discuss example 2-4. Model example 2-5. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html , Practice Questions for chapter 2, and model questions 6, 9, 12 & 13with class Quiz: Questions 12, 14, 18 & 19, pp. 41-42 Quiz: Problems 51-53, 55, 57, 59, 13 and 16, pp. 43-45 Lesson 2.5 (Motion at Constant Acceleration) Read pp 26-28. Model derivation of the equations of motion for constant acceleration from algebra. Model example 2-6, p. 28. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html , and do Physlet problems 6-11 with class. Quiz: Problems 18- 26 ,28 & 60, pp 43-46 Lesson 2.6A (Kinematics in One Dimension—Solving Problems) Short Quiz (Unit 2 Quiz 1). Review kinematic equations for constant acceleration, p. 28. Read closely text on problem solving, p. 29. Model examples 2-7 to 2-9, pp. 30-32. Demonstrate displacement using motion sensor (MBL). Go over selected quiz problems from this week: Problems 1-5, 7, 9, 10, 11, 51-53, 55, 57, 59, 13 and 16, 18- 26 ,28 & 60, pp 43-46. Homework: Go to the Prentice Hall Giancoli Physics website, http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html for “practice problems”. Write up problems 1-13 on a separate sheet and answer each problem showing your reasoning behind your answer. This assignment is due 10/4/04 Homework: Problems 27, 29, 31, 61 and 63, pp. 43-46, due 9/28/09 Lesson Plans: Physics Monday 9/19/05 Lesson 2.6B (Kinematics in One Dimension—Solving Problems) If you have not been graded on last week’s quiz, submit your work for a grade first thing today. Driving—Safe Distance and DUI: Go over problem 28, p. 44 and discuss safe driving distance and DUI. Go over selected homework from last week: Problems 27, 29, 31, 61 and 63, pp. 43-46. Tuesday 9/20/05 Wednesday 9/21/05 Thursday 9/22/05 (Block, 5th period) Friday 9/23/05 Homework: Read section 2-7, Falling Objects, pp. 32-38 for Tuesday, 9/20/05 Bring your textbook to class on block day (Thursday) Lesson 2.7A (Falling Objects) Demonstrate that a ball and paper fall at the same rate. Model examples 2-11 to-13 pp. 34-36. Model example 2-15, p.37. Model Problem 49, p.45 Homework: Questions 15-17, pp. 41-42—due 9/21/05 Homework: Problems 34-36, 41 43 and 46, p.44, due 9/21/05 Lesson 2.7B (Falling Objects) Read closely in class summary p.41. Go over selected homework problems from last night: : Problems 34-36, 41 43 and 46, p.44 HEADS UP!!! There will be a Unit 2 Exam on Thursday 9/22/05. Bring calculators and text books to school. We will start Unit 3, Kinematics in Two Dimensions, on Monday 9/26/05 Unit 2 Exam Using a motion sensor, demonstrate displacement, velocity and acceleration in class. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html , Chapter 2, Physlet problem 5 to demonstrate a ball thrown upward. Solve the problem at the board. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter2/deluxe.html , Puzzles for chapter 2, and model the question “Hang Time” with class Lesson Plans: Physics Monday 9/26/05 Lesson 3.1-3.3 (Kinematics in Two Dimensions—Vectors and Scalars, Adding and Subtracting Vectors, Multiplying a vector by a Scalar) Turn in Chapter 2 Test due today. Read pp. 48-52. Discuss vectors, scalars and, resultants of vectors using displacement vectors. Model both the “tail-to-tip” and “parallelogram” methods of adding and subtracting vectors. Go over questions 1-4, p. 70. Model problem 2, p.70. Tuesday 9/27/05 Do Physlet problems 1-2, in thttp://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html on monitor with the class to illustrate addition and subtraction of vectors. Homework: Go to the Prentice Hall Giancoli Physics website, http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html for “practice questions”. Write up problems 1-25 on a separate sheet (or print the pages)and answer each question showing your reasoning behind your answer. This assignment is due 10/1104 Homework: Questions 1-10, p. 70, due 10/3/05 Homework: Problems 1, 3-7, p.70, due 10/3/05 Homework: Read Appendix A-8 (Trigonometric Functions and Identities) Bring your textbook to class on block day (Thursday) Read pp.48-57 (Chapter 3, Kinematics in Two Dimensions: Vectors) for Wednesday, 10/6/04 Reread Appendix A-8 (Trigonometric Functions and Identities), pp. 1043-1045, for the next block period. Lesson 3.4 (Adding Vectors by Components) Review addition and subtraction of vectors. Model resolving vectors into orthogonal components Do Physlet problems 3-4, in the above referenced web-site http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html on monitor with the class to illustrate resolution of vectors into orthogonal components. Review the Pythagorean theorem and the trigonometric quantities associated with right triangles. Model trigonometric identities and model how trigonometric functions and identities can be used to find the components of a vector. Read pp. 52-57. Model example 3-1, p. 55. Closely read and list steps for problem solving when adding vectors, p. 56. Model example 3-2, pp. 56-57. Wednesday 9/28/05 Thursday 9/29/05 Friday 9/30/05 Class will determine displacement vectors, addition and subtraction of vectors, and multiplication of vectors by a scalar using string, tape measure, paper plates and protractors. Homework: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html ,MCAT Study Guide for chapter 3, and do questions 1-12, due 10/3/05 Homework: Problems 11-16, p.71, due 10/3/05 Read pp. 57-63 for Wednesday, 9/28/05 Lesson 3.5-3.6A (Projectile Motion) Short Quiz (Unit 3 Quiz 1). Model the motion of a ball thrown directly upwards graphically via a plot of its vertical position vs. time. Remark on the affect of the force of gravity. Demonstrate the difference for motion at right angles to the gravitational field. Superimpose the two motions via tables 3-1 and 3-2, p. 60. Read closely “Problem Solving for Projectile Motion”, p. 61. Model examples 3-3 & 3-4 pp. 6162. HEADS UP!!! On Friday 9/30/05, we will continue to discuss projectile motion and will introduce relative motion. There will be a short quiz (Unit 3 Quiz 2) on Friday, 9/30/05. Read pp. 63-69 for Monday, 10/11/04 Lesson 3.5-3.6B (Projectile Motion) Short quiz (Unit 3 Quiz 2). Model figures 3-18, 19 & 20 showing components of motion parallel and perpendicular to the gravitational field. Superimpose “x” and “y” equations of motion via tables 3-1 and 3-2, p. 60. Read closely “Problem Solving for Projectile Motion”, p. 61. Model examples 3-3 , 3-4 pp. 61-62. Model example 3-7 p. 64. THERE WILL BE A UNIT 3 EXAM ON WEDNESDAY, 10/5/05 (continued on back) Homework: Do problems 19-22, p. 72, due 10/3/05 Homework: Write the derivation of y=ax-bx2 as it is given in section 3-7, p. 66. Include a table defining all the variables you use in the derivation, due 10/3/05. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html, and do Physlet problems 3-6 Read example 3-8, p. 67, and pp.66-69 for Monday, 10/3/05 Homework: Try to solve problems 55, 56, 58, 63 and 65 p 75, and problem 71, p. 76, for Monday, 10/3/05 Lesson Plans: Physics Monday 10/3/05 Tuesday 10/4/05 Wednesday 10/5/05 Thursday 10/6/05 (Block, 5th period) Friday 10/7/04 Lesson 3.8 (Relative Velocity) Read pp. 66-69. Discuss Fig. 28, p. 67 and conceptual example 3-9.Model examples 3-10 to 3-12 pp. 68-69. Review vector notation, vector addition and subtraction. Read closely chapter summary, p.69, in class. Do Physlet problems 1-2, in the above referenced web-site http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter3/deluxe.html on monitor with the class to illustrate addition and subtraction of vectors. THERE WILL BE A UNIT 3 EXAM ON WEDNESDAY, 10/05/05. Homework: Questions 11-15, p. 70, due 10/04/05 Homework: Problems 40, 45, 49, 51, pp.73-74, due 10/04/05 Bring your textbook to class on block day (Thursday) Chapter 3 Review and Assessment Model problems 55, 56, 58, 63 and 65 p 75, and problem 71, p. 76. Go over selected homework problems UNIT 3 EXAM Bring your textbook to class on block day (Thursday) Silent Reading: read pp. 77-86 on Force (30 min.) Handout answer key to Unit 3 Exam (10 min.) Break (10 min.) Unit 3 exam makeup—all students can elect to take this exam. (40 min.) Lesson Plans: Physics Monday 10/10/05 Tuesday 10/11/05 Silent Reading: read pp. 77-86 on Force (30 min.) Complete unit 4 objectives quiz Lesson 4.1 (Force) Read pp. 77-78. Brainstorm: Why do objects move as they do? For examples, acceleration, deceleration and circular motion don’t happen by themselves. Force is required. Wednesday 10/12/05 Thursday 10/13/05 (Block, 6th period) Measuring force: The class will determine the magnitudes of forces using spring scales and other devices. The direction of force will be mapped for instances of pushing and pulling forces using makeshift compasses from paper plates and string. Introduce the Newton (the SI unit of force). Force vectors will be determined from these measurements. Forces will be classified as balanced or unbalanced. Discuss, define and observe “inertia”. Discuss whether a force is a vector or a scalar quantity. Homework: Questions 1, 3-6, p. 103, due 10/12/05 Lessons 4.2 & 4.3 (Newton’s First Law of Motion and Mass) Read pp. 78-80. Discuss inertia and the law of inertia. Discuss inertial and noninertial reference frames. Discuss friction. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter4/deluxe.html, Practice Questions. Review questions 1-4 to illustrate how to set up problems involving forces. Discuss mass as a measure of inertia. Discuss and compare the kilogram (SI), gram (cgs) and slug (English system). We shall study forces using lab equipment on Thursday during block period. Bring your textbook to class on block day (Thursday) Lesson 4.4 (Newton’s Second Law of Motion) Read pp. 80-82. Model examples 4-1 and 4-2 on p. 82. Brainstorm: What is the relationship between acceleration and force? Animated Problems: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter4/deluxe.html,, Physlet Problems 1, 2 and 3. Discuss and illustrate a “momentary” force. Force as a vector: Write the second law of motion as a three dimensional vector. Point out that three items are required to make a vector three dimensional. Those items may be its position coordinates (x,y,z) or its magnitude (r) and direction angles (polar angle, , and azimuth angle, ). Units of Force: Discuss the Newton (SI), Dyne (cgs) and pound (English system). Remember to use only one system when solving a particular problem. Homework: Questions 11-15, pp. 103-104, due 10/25/04 Homework: Problems 1-7, p. 104, due 10/25/04 HEADS UP!!! On Monday, 10/25/04, we will continue to discuss force Read pp. 83-86 There will be a brief quiz on objectives of chapter 4 on Monday too. Forces Friday 10/14/05 Study Forces using Foss Kits Study Forces using the ProbeMaster and appropriate probe(s) Homework: Write up your observations and analyze the results Lesson Plans: Physics Monday 10/17/05 Lessons 4.5 & 4.6 (Newton’s Third Law of Motion and Weight) Read pp. 83-89. Discuss load and reaction. Discuss friction and Newton’s third Law. Using the photogate and pulley , demonstrate weight and normal force via an Atwood’s machine. Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter4/deluxe.html, Practice Questions. Review questions 3-4 to illustrate how to set up problems involving forces. Bring your textbook to class on block day (Thursday) Tuesday 10/18/05 Wednesday 10/19/05 Review and Assessment Warm-up: All students at the board!! Do problems 58 and 62 (5 minutes) Answer questions 1-15, pp 103-104 in class Model problems 4, 6 and 7 p. 104 There will be a brief quiz on Tuesday, 10/18/05. Lesson 4.7 (Vector Forces and Free Body Diagrams) Model example 4-7, p. 90. Review vector addition and apply it to free-body diagrams. Closely read box entitled “Problem Solving—Newton’s Laws; Free-Body Diagrams” on p. 91. Unit 4 Quiz 1 –will include one problem on two dimensional kinematics (10 minutes) All students at the board—class to write examples 4-9, 4-10 4-11 and 4-13 pp. 92-95 at the board simultaneously. Then, the responsible student will explain the example. Homework: Write up your observations and analyze the results Homework: Questions 1, 3-6, 11-15, 16-22 pp. 103-104, due 10/24/05 Homework: Problems 1-7, 12-14, 19, 27, 28, 32 and 36 pp. 104-107 , due 10/24/05 Lesson 4.8 (Applications Involving Friction and Inclines) Read pp. 96-102. Model examples 4-16 and 4-17 on pp. 99-100. Warm-up: What is Friction? Observe: Does a 2N force have the same, less or greater friction than a 4N force? Try this on an inclined plane. Brainstorm: In which direction is the friction force? How is friction related to the normal force? Discuss the difference between static and kinetic friction. Review table 4-2, p. 97, Coefficients of friction. Animated Problems: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter4/deluxe.html,, Physlet Problems 8 and 9. Work out the solutions at the board. Thursday 10/20/05 (Block, 6th period) Homework: Problems 61, 62 and 63, p. 110 HEADS UP!!! On Thursday 10/21/05, we will conclude the discussion on force Read pp. 102-103. Review and Assessment Warm-up: All students at the board!! Do problems 61, 62 and 63, p.110 (5 minutes) Read closely box on “Problem Solving” on p. 102 Read closely Chapter 4 summary on p. 103 Do problem 72 on p. 110 Using a dual force sensor (to establish the applied force for several combinations of 200 kg masses), a photogate and pulley, plot the coefficient of static friction and kinetic friction vs. applied force between a textbook’s surface and a wood surface.per example 4-16. The plot should resemble fig. 4-28, p.98. Friday 10/21/05 There will be a unit 4 test on Monday, 10/24/05 Lesson Plans: Physics Monday 10/24/05 Tuesday 10/25/05 Unit 4 test—Complete this and hand it to the instructor Do in class problems on the instruction work sheet Homework: read pp. 112-119 for Tuesday, 10/25/05 Lessons 5.1 and 5.2 (Circular Motion; Gravitation—Kinematics and Dynamics of uniform circular motion) Wednesday 10/26/05 Derive kinematic formulas for the magnitude of the radial acceleration vector. Calculate the radial acceleration of the rim of a pulley attached to a book movig at constant velocity, photogate and force sensor Model examples 5-1 and 5-2, pp. 114-115 Derive dynamic formulas for the force in the radial direction for uniform circular motion Measure the force required for uniform circular motion using the force sensor and a satellite of know mass attached to the sensor and revolving horizontally (example 5-3) and then vertically (example 5-5). Animated Problems: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter5/deluxe.html,, Physlet Problems 1, 2 and 3. Work out the solutions at the board. Homework: Write up your observations and analyze the results Homework: Read pp. 119-121 for Wednesday 10/26/05 Homework: Questions 1, 4, 5 and 9 p. 138, due 10/31/05 Homework: Problems 1-6, 8, 10 and 12 pp. 139-140 , due 10/31/05 Lessons 5.3A and 5.4A (Circular Motion; Gravitation—A car rounding a curve and non-uniform circular motion) Read pp. 119-121. Model examples 5-7and 5-8on pp. 120-121. Warm-up: What is the inward force that causes a car to turn in a curve? Animated Problems: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter4/deluxe.html,, Physlet Problems 8 and 9. Work out the solutions at the board. Homework: Questions 2, 3, 11,and 13 p. 140 , due 10/31/05 Thursday 10/27/05 Lessons 5.3B and 5.4B (Circular Motion; Gravitation—A car rounding a curve and non-uniform circular motion) ·Warm-up: All students at the board!! Do problems 1-6, 8, 10 and 12 pp. 139-140 (15 minutes) Friday 10/29/05 Observe: Excerpt from DVD on race car driving techniques for skidding on a curve Brainstorm: How is the driver controlling the turning of the car? Discuss what happens when the net force in circular motion is not directed towards the center of the circle. Homework: Questions 14-18, p. 139, due 10/31/05 Homework: Problems 7, 9, 11, 13, 14, 16 and 21, pp. 139-141, due 10/31/05 Homework: Problems 22, 23, 24, p. 141, due 10/31/05 Read pp. 122-127 on “centrifugation” and Newton’s Law of Universal Gravitation for Monday, 10/31/05 Lesson Plans: Physics Monday 10/31/05 Tuesday 11/1/05 Wednesday 11/2/05 Thursday 11/3/05 Lesson 5.6 (Newton’s Law of Universal Gravitation) Model examples 5-11, 5-12 and 5-13, pp. 126-127. Discuss how the gravitational force can be used to slingshot a space ship. Animated Problems: Go to http://cwx.prenhall.com/bookbind/pubbooks/giancoli/chapter5/deluxe.html,, Physlet Problems 4, 5 and 6. Work out the solutions at the board. CAHSEE CAHSEE Lesson 5.7 (Gravity Near the Earth’s Surface: Geophysical Applications) Read pp. 127-129. Model example 5-14, p. 128. Friday 11/4/05 Demonstrate how to measure the gravitational constant “g” using a balance and the force sensor. Discuss ways to measure or research and find the radius of the earth. Using the measured gravitational constant and a best guess of the radius of the earth at the classroom, estimate the mass of the earth. Homework: Problems 25, 26, 27 and 36, p. 141, due 11/7/05 Homework: read pp. 129-137 for Monday, 11/7/05 and Tuesday, 11/8/05. There will be a Unit 5 Test on Wednesday, 11/9/05 Lesson Plans: Physics Monday 11/7/05 Lessons 6.1-6.2 (Work and Energy—Work done by a Constant Force and by a Varying Force) Read pp. 145-150. Model examples 6-1 and 6-2, pp. 147-148. Tuesday 11/8/05 Define “Work” Lab Report No. 1 Using the force sensor and photogate, have students tabulate displacement and force data, graph the data of force (parallel to displacement) vs. displacement and, calculate the work required to drag a book across a table when the force is in line with the displacement and when the force is at a variable angle to the displacement. Due 12/6/04 Homework: Questions 1-7, p. 172, due 11/14/05 Homework: Problems 1-11 (odd), 12-16 (even), due 11/14/05 Lessons 6.3-6.4 (Kinetic and Potential Energy) Read pp. 150-157. Model example 6-4, 6-5, 6-6 and 6-7, pp. 152-155. Wednesday 11/9/05 Derive the equations for work done and translational kinetic energy from 1-dimensional kinematics, Newton’s second law, and the definition of “Work” Discuss the work-energy principal Derive the equations for work done by an external force and gravitational potential energy from 1-dimensional kinematics, Newton’s second law and the definition of “Work” Discuss Hooke’s law (the spring equation) and derive the equation for the potential energy associated with elastic materials. Homework: Questions 8-12, pp. 172-173, due 11/14/05 Homework: Problems 17-28 (odd), 29, 30 and 31, due 11/14/05 Lessons 6.5-6.6 (Conservative and Non Conservative Forces; Mechanical Energy and its Conservation) Thursday 11/10/05 A conservative force is one for which the work done does not depend on the path taken, but only on the initial and final positions. A non-conservative force is one for which the work done depends on the path taken. The net, or total, work done is the some of the work done by the conservative forces and the non-conservative forces. Homework: Questions 13-15, p. 173, due 11/14/05 Homework: Problems 32-37, pp. 175-176, due 11/14/05 Lesson 6.7A (Problem Solving Using Conservation of Mechanical Energy) Read pp. 159-165. Model examples 6-8, 6-9, 6-10, 6-11, 6-112, 6-13 and 6-14, pp. 160-165. Conservation of Mechanical Energy: If only conservative forces are acting, the total mechanical energy of a system neither increases nor decreases in any process. It stays constant. Review and Assessment: Chapter 6 Work and Energy; Sections 6-1 to 6-4 Friday 11/11/05 Quiz: Problems 9, 13, 17 and 29 , pp. 174 and 175 (25 min) Go over Problems 11, 14, 18, 26 and 28 p.175 (20 min.) Homework: Questions 16-20, p. 173, due 11/14/05 Homework: Problems 38-48 (even), p. 176 due 11/14/05 Lesson Plans: Physics Monday 11/14/05 Lesson 6.7B (Problem Solving Using Conservation of Mechanical Energy) Read pp. 159-165. Model examples 6-8, 6-9, 6-10, 6-11, 6-112, 6-13 and 6-14, pp. 160-165. Tuesday 11/15/05 Wednesday 11/16/05 Thursday 11/17/05 Friday 11/18/05 Go over : Problems 17-28 (odd), 29, 30 and 31, p. 174 Quiz: Answer questions on back of lab about the efficiency of an inclined plane (15 min) THERE WILL BE CHAPTER 6 TEST THIS THURSDAY Lab Report No. 1 Using the force sensor and photogate, have students tabulate displacement and force data, graph the data of force (parallel to displacement) vs. displacement and, calculate the work required to drag a book across a table when the force is in line with the displacement and when the force is at a variable angle to the displacement. Due 11/21/05 Lessons 6.8-6.10 (Energy Conservation and Power) Go over Problems 38-48 (even), p. 176 (20 min.) Review the Law of Conservation of Energy and model example 6-15, p. 168 (10 min) Define Power and model example 6-18, p. 171 (5 min) Read Summary on p. 172 (10 min) Homework: Problems 49-70 (every third problem), pp. 177-178, due 11/28/05 THERE WILL BE CHAPTER 6 TEST ON WEDNESDAY. Review and Assessment: Chapter 6 Work and Energy; Sections 6-5 to 6-10 Quiz: problems 38-48 (even), p. 176 (25 min) Go over Problems 32-37, pp. 175-176 (20 min) Go over general problems 73, 74, 80 and 84 , pp. 177-179 (15 min) Chapter 6 Test Lesson Plans: Physics Monday 11/21/05 Tuesday 11/22/05 Wednesday 11/23/05 Thursday 11/24/05 Thanksgiving Holiday Friday 11/25/05 Lesson Plans: Physics Monday 11/28/05 Lessons 7.1-7.3 (Linear Momentum , Conservation of Momentum and, Collisions and Impulse) Tuesday 11/29/05 Wednesday 11/30/05 Thursday 12/1/05 Friday 12/2/05 Define Linear Momentum and model example 7-1, p. 182 (10 min.) Define the Law of Conservation of Linear Momentum and model examples 7-3, p. 184, and 7-4, p. 185 (15 min.) Define Impulse and model example 7-5, p. 186 (15 min.) PROJECT: Linear Momentum—What Is It? Day 1 (20min.) Homework: Problems 1-20 (even), pp. 202-203, due 12/5/05 Lessons 7.4-7.7(Collisions) Define Elastic Collisions and model examples 7-6, p. 189 and 7-7, p. 190 (15 min.) Define Inelastic Collisions and model example 7-9, p. 191 (10 min.) Discuss the vector nature of momentum and model example 7-10, p. 193 (10 min.) PROJECT: Linear Momentum—What Is It? Day 2 (20min.) Homework: Problems 21-45 (every fourth problem), pp. 203-205 due 12/5/05 Lessons 7.8-7.10 (Center of Mass) Define Center of Mass and model example 7-11 p. 196 (15 min.) Define the total linear momentum of a system of particles as the product of the total mass and the velocity of the CM of the system and model problem 57, p. 206 (15 min.) Read Summary on p. 201 (10 min) PROJECT: Linear Momentum—What Is It? Day 3 (5min.) Homework : Problems 46-60 (every fourth problem), pp. 206-207 due 12/5/05 Do lab on Projectile Motion. Lab Report No. 1. Due 12/5/05 (40 min.) Watch presentation “Linear Momentum” (15 min.) PROJECT: Linear Momentum—What Is It? Day 4 (45min.) THERE WILL BE A TEST OF CHAPTER 7 ON MONDAY. Lesson Plans: Physics Monday 12/5/05 Tuesday 12/6/05 Chapter 7 Test Lessons 8.1-8.3 (Rotational Motion—Angular Quantities; Kinematic Equations for Uniformly Accelerated Rotational Motion and; Rolling Motion) Review the meaning of “a revolution of a wheel” (1/period = 1/T= frequency= f =1 rev/s = 2 rad/s); “radians” (=l/r); tangential acceleration (atan =v/t) and ; radial acceleration (ar = v2/r) (5 min) Assessment: Each student will do in class problems 1, 6 and 7, p. 234 (10min) Discuss angular velocity (=rad/st=(1/r) ( Wednesday 12/7/05 Thursday 12/8/05 and angular Homework: Problems 11, 12 & 24 pp. 234-235 due 12/7/05 You will solve these problems at the board tomorrow. Lessons 8.4-8.6 (Rotational Motion—Torque and Rotational Dynamics) Collect homework (1 min.) Students at the board: Solve Problems 11, 12 & 24 pp. 234-235 (5 min) Demonstrate moment arm using the door. Define torque (rF). (3 min) Assessment: Each student will do in class problems 29 and 30 p. 235 (10 min) Since = mr2 from =rF=r matan =rm(r), and if the moment of inertia is defined as I= mr2, then = Iwhich is Newton's second law for rotation. Demonstrate moment of inertia using rod and string (10 min) Model sample problem 8-6 p. 222 (3 min) Assessment: Each student will do in class problems 34 and 35 p. 236 (10 min) Model example 8-12, p.224. PAY ATTENTION as we will be doing pulley experiments this week. (5 min) Assessment: Each student will do in class problem 40, p. 236 (10 min) Homework: Problems 33, 37 and 44 , p. 236 due 12/8/05. You will solve these problems at the board tomorrow. Lessons 8.7-8.9 (Rotational Motion—Rotational Kinetic Energy; Angular Momentum and Angular quantities as vectors)) Collect homework (1 min.) Students at the board: Solve Problems 33, 37 and 44 , p. 236 (5 min) Since translational KE = (mv2/2), and ar = v2/r=(r)2/r=r, then rotational KE = ( (mr2))2/2=I2/2. The total KE is the sum of translational and rotational KE. (5 min) Model example 8-14, p. 226 (5 min) Assessment: Each student will do in class problems 50 and 51, p. 237 (10 min) The work done on a body rotating about a fixed axis, W =F ( Friday 12/9/05 l/t) =v/r=2f) acceleration (t=rad/s2=(1/r)(v/t)=atan/r). Note that radial acceleration, ar, is related to the angular velocity (ar = v2/r=(r)2/r=r) (5 min) Assessment: Each student will do in class problems 8 9 and 20, pp. 234-235 (10 min) Model example 8-5, p. 214 (3 min) List the kinematic equations for constant angular acceleration, p. 215 (3 min). Model example 8-6, p.215 (3 min) Model example 8-7, p. 216 (3 min) Assessment: Each student will do in class problems 10 and 22 pp. 234-235 (10 min) l)=Fr((is useful for analysis of systems with pulleys (3 min) Since the angular momentum is defined as L=I then = It)=L/t (conservation of angular momentum) Model example 8-16, p. 230 (10 min) Assessment: Each student will do in class problems 57 and 58, p. 238 (10 min) Rotational Motion Lab: (45 min.) THERE WILL BE A CHAPTER 8 TEST ON MONDAY Lesson Plans: Physics Monday 12/12/05 Tuesday 12/13/05 Chapter 8 Test Lessons 9.1-9.3 (Bodies in Equilibrium--Statics) Review the vector resultant force: Fnet=Fi ,and: torque, =Fr. Counter-clockwise (CCW) torques are positive by convention in this course. (3 min.) Assessment: Each student will do in class problems 1 and 3, p. 266 (10min) Discuss the two conditions for equilibrium, i.e., Fi = 0 and = 0 = I. (5 min) Assessment: Each student will do in class problems 18 and 20, p. 268 (10 min) Demonstrate the lever principle using the force sensor and a scale. (10 min) Model example 9-8, p. 248 (3 min) Assessment: Each student will do in class problems 22 and 26 p. 268 (10 min) Homework: Problems 12 & 25 pp. 267-268 due 12/14/05 You will solve these problems at the board tomorrow. Lessons 9.6-97 (Bodies in Equilibrium—Elasticity and Fracture) Wednesday 12/14/05 Collect homework (1 min.) Students at the board: Solve problems 22 and 26 p. 268 (10 min) Chapter 8 test: Do General Problem 76, p. 239 (10 min) Elongation of a beam, L, occurs because the beam is elastic and a force, F, is pulling on it. Hooke’s law relates the force to the elongation as follows: F=kL, where k = EA/Lo . See table 9-1, p. 254, for values of the elastic moduli, E, for several materials. (3 min) Assessment: Each student will do in class problems 44 and 45 p. 271 (10 min) Thursday 12/15/05 Since F/A=EL/Lo , the ultimate strengths of materials in tension, compression and shear determine if an object fractures or breaks. See table 9-2, p. 258, for ultimate strengths of several materials. (3 min) Model sample problem 9-13 p. 258 (3 min) Assessment: Each student will do in class problems 55 and 58 pp. 271-272 (10 min) Model problem 72, p. 273 (8 min) Homework: Problems 49 and 50 , p. 271 due 12/15/05. You will solve these problems at the board tomorrow. Lesson 9.8 (Bodies in Equilibrium—Arches and Domes) Friday 12/16/05 Collect homework (1 min.) Students at the board: Solve problems 49 and 50 , p. 271 (5 min) Compare the semicircular and pointed (Moorish) arches shown in fig. 9-35. What advantages does the pointed arch have over the semicircular arch? (5 min) Assessment: Each student will do in class problem 62, p. 272 (5 min) A dome is a three-dimensional arch and is most statically stable when under compression. Model example 9-15, p.264 (10 min) Assessment: Each student will do in class problem 63, p. 272 (10 min) THERE WILL BE A CHAPTER 9 TEST ON 1/3/06 Lesson Plans: Physics Monday 1/2/06 Tuesday 1/3/06 HOLIDAY Wednesday 1/4/06 Lessons 10.1-10.4 (Fluids—Density, Pressure (absolute and gauge) and Pascal’s Principle) Chapter 9 test: (60 min) Density of an object is mass per unit volume: = m/V . Discuss table 10-1, densities of substances, p. 276. Model example 10-1, p. 276 (5 min) Assessment: Each student will do in class problems 1 2 and 4, p. 304 (10 min) Fluids exert pressure in all directions. The pressure applied to a confined fluid increases the pressure throughout by the same amount (Pascal’s Principle). The pressure of a column of fluid with cross sectional area, A, and height, h, is P Thursday 1/5/06 =(V)g/A =(Ah)g/A=gh . Model example 10-2, p. 278. (5 min) Assessment: Each student will do in class problems 7, 8 and 16 p. 304 (15 min) The absolute pressure, P, is the sum of the atmospheric pressure, P A, and the gauge pressure, PG: P = PA + PG. (1atm=1.013N/m2 = 101.3 kPa = 14.7 psi). (3 min) Assessment: Each student will do in class problems 9, and 10 p. 304 (7 min) ·Homework: Problems 15 and 17 , p. 304 due 2/17/05. You will solve these problems at the board tomorrow. Lessons 10.5-10-6 (Fluids—Measurement of Pressure, Buoyancy) Friday 1/6/06 = F/A= mg/A= Students at the board: solve Problems 15 and 17 , p. 304 (10 min) Manometer demonstration: The open-tube manometer measures the difference in pressure between two pressures. P = P2-P1=gh (3 min) Handout TM 48A-- PUT THIS IN YOUR NOTEBOOKS (1-min) Assessment: Each student will measure the barometric pressure using the pressure probe. Then each student will determine the pressure exerted on an open-tube manometer from a syringe attached to one end by measuring the difference in fluid height, h , on the U-tube. In order to do this, the density of the fluid in the U-tube will also be measured using a balance and calibrated cylinder. (15 min) Archimedes’ Principle is this: the buoyant force on a submerged object is equal to the weight of the fluid displaced by that object. When the weight of the fluid that is displaced is greater than the weight of the object, then the object will float (i.e., rise) through the fluid. Otherwise, it will sink: FB = (mfluid)g= (fluidVobject)g ( 3 min.) Assessment: Each student will do in class problem 23, and 24 p. 305. (10 min) THERE WILL BE A CHAPTER 10 TEST NEXT TUESDAY Density and Specific Gravity—what are they and how do they differ? "The weight of the matter", Pressure—what is it? Atmospheric Pressure and Gauge Pressure—when do I use them? Pascal and Arcimedes both had Principles—what are they and how are they related? How do they make Barometers? How do they work and what do they measure? How can I use Archimedes Principle as an investigative tool? Lesson Plans: Physics Monday 1/9/06 Lessons 10.7-10.9 (Fluids—Fluids in Motion and the Bernoulli Equation) Laminar flow in a tube or pipe of cross-sectional area A: A fluid flowing with velocity, v., will transport mass at the following rate: m/t=V/t=Al/t=Av. Model example 10-11, p. 289 (10 min) Assessment: Each student will do in class problems 35 and 36, p. 305 (10 min) Continuity: Mass flow rate,m/t, in laminar flow is constant regardless of changes in the cross-section of the flow channel; changes in fluid density or; changes in velocity. Model example 10-10, p. 288 (5 min) Assessment: Each student will do in class problem 34 p. 305 (5 min) Tuesday 1/10/06 TEST ON CHAPTER 10 Wednesday 1/11/06 Lessons 11.1-11.3 (Vibrations and Waves—Simple Harmonic Motion) Thursday 1/12/06 Bernoulli’s Equation: At every point in a fluid, P + v2/2 + gy = constant based on the conservation of energy within the fluid. Model example 10-12, p. 291. Model use of the Bernoulli equation for spigots on an open tank and a pressure vessel. (15 min) Assessment: Each student will do in class problems 37, and 38 p. 306 (15 min) ·Homework: Problems 40 and 42 , p. 306 due 1/10/06. Review: The spring equation is F = -kx. Model example 11-1, p. 311. Show conceptually how opposing forces results in simple harmonic motion. Define: displacement (x), amplitude (xmax), cycle, period (T) and frequency ( = 1/T) (10 min) Assessment: Each student will do in class problems 1,2 and 3 p. 342 (10 min) The total energy of a simple harmonic oscillator, E, is the sum of the potential energy and the kinetic energy: E= mv2/2 +kx2/2. E is constant. Model example 11-3. (15 min) Assessment: Each student will determine v as a function of displacement, x, relative to amplitude xmax. (5 min) If xmax is the radius of a circle, what is the value of the sin of for any point on the circumference (x, y)? Demonstrate the relation between uniform circular motion and simple harmonic motion using the lazy susan. Note the period of revolution, T=2xmax/vo. Show that for simple harmonic motion T=2(m/k)0.5 (15 min) Lessons 11.4-11.8 (The Pendulum, Damped Harmonic Motion, Forced vibrations/Resonance and Waves) Students at the board: solve Problems 4 , 6 and 27 , pp. 342-343 (10 min) Pendulums: Model example 11-8, p. 320. Demonstrate using pendulums of different lengths. Discuss and demonstrate damping of and resonance in SHM..(15min) Assessment: Each student will do in class problems 28 , 29 and 30, p. 343 (5 min) Wave Motion: Define amplitude, wavelength () and wave velocity (v). For a string in tension, FT =-kx, the total energy is E= mv2/2 + kx2/2= mv2/2 -FTx/2=0 which implies that .v=(FT /m/x)0.5 .. Model example 11-10, p. 326. (5 min) Assessment: Each student will do in class problem 34, 35 and 38 pp. 343-344 (5 min) This relation can be generalized to v= (E/)0.5 for solids, where E is the elastic modulus. Model example 11-11 p. 328. (5 min) Assessment: Each student will do in class problems 37, 40 and 42 p. 344 (15 min) Homework: Problems 31, 36 and 41 , pp. 343-344 due 1/17/06 THERE WILL BE A CHAPTER 11 TEST NEXT THURSDAY Friday 1/13/06 Bernoulli's Equation and Conservation of Energy, how are they related? How can Bernoulli's Equation be used to explain the lift of an Airplane Wing , the drive of a Sail, and similar phenomena? Lesson Plans: Physics Monday 1/16/05 Tuesday 1/17/06 HOLIDAY Lessons 11.9-11.10 (Energy Transported by Waves and Intensity) Students at the board: solve Problems 31, 36 and 41 , pp. 343-344 (10 min) I=Intensity =Power/Area=(Energy/time)/Area=( kxmax2/2)/time/area for a two-dimensional wave. If the wave propagates spherically, the area = 4r2, where r is the radius of the 2 Wednesday 1/18/06 Thursday 1/19/06 sphere. Therefore, I is proportional to xmax / r2 . Model example 11-12, p. 330. (15 m) Assessment: : Each student will do in class problem 46 p. 344 (5 min) Intensity as a function of period (T) and amplitude (xmax): Since k=42m/T2 , then Energy=E= kxmax2/2=22 mxmax2 /T2 Since m=V=(area)(v)(time), then I=Intensity = (Energy/time)/Area=22 vxmax2 /T2 (10 min) Assessment: Each student will do in class problem 48 p. 344 (5 min) Lab (Simple Harmonic Motion) Pre-lab (How can angle be represented as a function of lapsed time, t, and period,T?) (5 min) Lab: Using EXCEL, determine the value of x at any time, t, for a simple harmonic oscillator consisting of mass, m, and spring with spring constant, k. Plot the motion as a function of time. Also determine the values of velocity and acceleration as a function of time and plot these. (40 min) Post-lab: What does sinusoidal mean? CHAPTER 11 TEST AND SEMESTER FINAL Friday 1/20/06 What is Simple Harmonic Motion and why is it important? How can I use Conservation of Energy to study this motion? Review for the Final Rotational Motion: Know that 1/period = 1/T= frequency= f =1 rev/s = 2 rad/s. Know the meaning of angular velocity (=rad/st=(1/r) ( l/t) =v/r=2f) and angular acceleration (t=rad/s2=(1/r)(v/t)=atan/r). Know what torque is ( = mr2 from =rF=r matan =rm(r)). Know what Newton's second law for rotation is (I= mr2, then = IKnow what rotational kinetic energy is Since translational KE = (mv2/2), and ar = v2/r=(r)2/r=r, then rotational KE = ( (mr2))2/2=I2/2. The total KE is the sum of translational and rotational KE). Bodies in Equilibrium—Statics: Know that the vector resultant force is: Fnet=Fi ,and: that the scalar torque is, =Fr. Counter-clockwise (CCW) torques are positive by convention in this course. Know the two conditions for equilibrium, i.e., Fi = 0 and = 0 = I. Know that the Elongation of a beam, L, occurs because the beam is elastic and a force, F, is pulling on it. Hooke’s law relates the force to the elongation as follows: F=kL, where k = EA/Lo , where “E” is the elastic modulus. Since F/A=E(L)/Lo , the ultimate strengths of materials in tension, compression and shear determine if an object fractures or breaks. Fluids: Know that Density of an object is mass per unit volume: = m/V . The pressure applied to a confined fluid increases the pressure throughout by the same amount (Pascal’s Principle). The pressure of a column of fluid with cross sectional area, A, and height, h, is P = F/A= mg/A= =(V)g/A =(Ah)g/A=gh . The absolute pressure, P, is the sum of the atmospheric pressure, P A, and the gauge pressure, P G: P = PA + PG. (1atm=1.013 x 105 N/m2 = 101.3 kPa = 14.7 psi). Archimedes’ Principle is this: the buoyant force on a submerged object is equal to the weight of the fluid displaced by that object. When the weight of the fluid that is displaced is greater than the weight of the object, then the object will float (i.e., rise) through the fluid. Otherwise, it will sink: FB = (mfluid)g= (fluidVobject)g. Know that for Laminar flow in a tube or pipe of cross-sectional area A, a fluid flowing with velocity, v., will transport mass at the following rate: m/t=V/t=Al/t=Av. Mass flow rate,m/t, in laminar flow is constant regardless of changes in the cross-section of the flow channel; changes in fluid density or; changes in velocity. This is called “continuity”. Use Bernoulli’s Equation: At every point in a fluid, P + v2/2 + gy = constant based on the conservation of energy within the fluid. Vibration and Waves: Know that the spring equation is F = -kx. The total energy of a simple harmonic oscillator, E, is the sum of the potential energy and the kinetic energy: E= mv2/2 +kx2/2. E is constant. Know that for simple harmonic motion, the period is T=2(m/k)0.5 . For a simple pendulum, When a mass, m, at the end of a string of length, L, is displaced by a vertical angle, , then the restoring force, F= -mgsin ~ mg, if is nearly 0 rad. Since =x/L, where x is the arc length of the pendulum arc, then, F= -mgx/L = kx. Therefore, T=2(m/k)0.5 = 2(L/g)0.5 . Define amplitude, wavelength () and wave velocity (v) of a wave. .. The period of a wave is T= v. For a string in tension, FT =-kx, the total energy is E= mv2/2 + kx2/2= mv2/2 -FTx/2=0 which implies that .v=(FT /m/x)0.5 This relation can be generalized to v= (E/)0.5 for solids, where E is the elastic modulus. Know that I=Intensity =Power/Area=(Energy/time)/Area=( kxmax2/2)/time/area for a two-dimensional wave. If the wave propagates spherically, the area = 4r2, where r is the radius of the sphere. Therefore, I is proportional to x max2 / r2 for a spherical wave. Lesson Plans: Physics Monday 1/23/06 Lessons 12.1-12.3 (Sound—Characteristics, Intensity and Amplitude) Students at the board: solve Problems 74 and 78 , p. 346 (10 min) Review: For a string in tension, FT =-kx, the total energy is E= mv2/2 + kx2/2= mv2/2 FTx/2=0 which implies that .v=(FT /m/x)0.5 . This relation can be generalized to v= (B/)0.5 for gases and liquids, where B is the bulk modulus (see table 9-1, p. 254 and note the correction below). (5 min) The speed of sound in air: v = (B/)0.5 = ( 1.42 x 105 N-m-2/1.29 kg-m-3)0.5 = 331 m-s-1 at STP. For other temperatures, “t” deg. C, the speed of sound in air is v = (331+0.58t). The speed of sound in helium is (892 + 1.57 t). (5 min) Assessment: Each student will determine the wavelength of a 50kHz ultrasonic sound wave in air at 40 degrees C. (10 min) Decibels: (in dB) = 10 log (I/Io).= the intensity level in decibels where Io = 10-12 W-m-2. Model example 12-3, p. 351. I is proportional to xmax2 / r2 for a spherical wave. Model example 12-4, p. 352. (15min) Assessment: Each student will do in class problem 10, p. 376 (10 min) Amplitude related to Intensity: Since k=42m/T2 , Energy=E= kxmax2/2=22 mxmax2 f2 Since m=V=(area)(v)(time), then I=Intensity = (Energy/time)/Area=22 vxmax2 f2. Model example 12-5, p. 352. (5 min) Tuesday 1/24/06 Assessment: Each student will do in class problem 20 p. 377. (10 min) Homework: Problems 2, 9 and 21 , pp. 376-377 due 1/24/06. You will solve these problems at the board tomorrow. THERE WILL BE A CHAPTER 12 TEST ON THURSDAY Lessons 12.5-12.6 (Sound—Sources) Students at the board: solve Problems 2, 9 and 21 , pp. 376-377 (10 min) Pressure vs. Displacement Waves: If a displacement wave is sinusoidal, the pressure wave is negatively sinusoidal, or out-of-phase by a “quarter wave” (i.e, /2 rad—see fig. 12-3, p. 350). Discuss nodes and antinodes of a tube open at both ends for which fn =v/n = nv/(2L) = n f1 , n=1,2,3,… (see fig. 12-12, p. 358). Demonstrate using a recorder. For a tube closed at one end, f2n+1 =v/2n+1 = (2n+1)v/(4L) = (2n+1) f1 , n=0,1,2,3,…(15 min) Wednesday 1/25/06 Assessment: : Each student will demonstrate and do in class problem 29 p. 377 (15 min) Homework: Problems 32 and 34 , p. 377 due 1/25/06 Lessons 12.7-12.10 (Sound—Interference, the Doppler Effect and Shock Waves) Students at the board: solve Problems 32 and 34 , p. 377 (10 min) Interference: Using Excel, make two sine waves of equal amplitude at 262 Hz (C) and 277 Hz (C#) and determine amplitudes for .001 second intervals for a duration of 0.20 seconds. Superimpose the two waves. Graph the resulting wave. Try playing two recorders at the same time to get interference. (20 min.) Thursday 1/27/06 Friday 1/28/06 Assessment: Each student will do in class problem 42 p. 378. (10 min) Doppler Effect: Discuss source in motion / stationary observer vs. observer in motion / stationary source. Model examples 12-13 and 12-14, p. 368. (20 min) Assessment: Each student will do in class problem 51 p. 378. (10 min) Sonic Boom: The Mach number is the ratio of the speed of an object to the speed of sound in the medium. Bow waves from a boat are shock waves relative to the speed of water waves it produces. See Fig. 12-24, p. 369 for explanation. (10 min) Assessment: Derive equation 12-5, sin () =vsnd/vobj (10 min) CHAPTER 12 TEST-To prepare, answer the questions on the back of this page. What are the Characteristics of Sound? o What is... Infrasound? Ultrasound? Pitch? Frequency? Loudness? Intensity? I have heard of Decibels. What are they and how are they related to Intensity? How is Wave Amplitude related to Intensity? How is the Ear constructed and how does it sense sound? What are the common sources of Musical Sounds? How do Vibrating Strings produce sounds of different frequencies? How do Vibrating Air Columns produce sounds of different frequencies? How does Quality effect how a particular instrument sounds? What are Beats and how do they arise from the Interference of sounds? We have all heard the train whistle change pitch as it passes us. Does the Doppler Effect explain this? What is a Sonic Boom and how is it produced? Lesson Plans: Physics Monday 1/30/06 Tuesday 1/31/06 Lessons 13.1-13.10 (Temperature and Kinetic Theory) Atomic Theory: 1 amu = 1.66 x 10-27 kg x atomic mass. Model example 13-1, p. 383 (5 min.) Assessment: Each student will do in class problems 1, 2 p. 412. (10 min) Temperature: K=C+273; F=1.8C + 32. Temperature is the measure that is used to determine thermal equilibrium between two or more systems. Thermal equilibrium occurs because heat no longer flows between the different systems. (5 min). Assessment: Each student will do in class problems 3, 8 pp. 412-413 (5 min) Thermal Expansion: L/Lo = T, where “” is the coefficient of linear expansion (see table 13-1, p. 388). Model example 13-5, p. 389. Similarly, V/Vo = T, where “” is the coefficient of volume expansion (5 min) Assessment: Each student will do in class problem 12 p.413 (5 min) Thermal Stresses: Recall that Elongation of a beam, L, occurs because the beam is elastic and a force, F, is pulling on it. Hooke’s law relates the force to the elongation as follows: F=kL, where k = EA/Lo , where “E” is the elastic modulus. Hence, F/A=E(L)/Lo. Since L/Lo = T, substitution into F/A=E(L)/Lo gives: F/A=ET. Model example 13-8, p. 392. (5 min.) Assessment: Each student will do in class problem 25 p.414 (5 min) The Ideal Gas Law: PV=nRT. This law adequately explains prior gas laws. The number of moles=mass/molar mass=m/M= N/NA. N=number of atoms and NA =Avogadro’s number= 6.02 x 1023. R=8.315 J/(mol-K)=.0821 L-atm/(mol-K)=Gas Law constant. Hence, PV=nRT= N(R/NA)T=NkT, where k= =Boltzmann’s constant= 1.38 x 10-23 J/K. Model problem 42, p. 414 (10 min) Assessment: Each student will do in class problem 43 p.414 (5 min) Homework: Problems 31 and 40 , p. 414 due 2/1/06. THERE WILL BE A CHAPTER 13 TEST ON THURSDAY Lessons: Chapter 13.11-13.14 (Temperature and Kinetic Theory) Students at the board: solve Problems 31 and 40 , p. 414 (10 min). Introduction to Chemical Thermodynamics:The kinetic theory requires that the average translational kinetic energy of molecules in a gas be directly proportional to temperature (KE = 1/2mv2=3/2kT). The greater the temperature of the object, the greater the energy of motion of the atoms and molecules that make up the object. Discuss the idea that all substances have energy of motion within them (for a monatomic ideal gas, U=N(1/2mv2)= 3/2NkT=3/2nRT) . Temperature reflects only the average kinetic energy of the individual molecules. The total of all of the energy of the molecules constitutes the internal energy of the substance. Model example 13-16 and 13-17, pp. 401 and 402. (10 min) Assessment: Each student will do in class problem 52 p.415 (5 min) Phases, Phase Equilibria and Phase Diagrams: Real atoms do have interactions. Liquid condenses from the gas and solids freeze from the liquid state due to intermolecular interactions. Condensation, deposition, freezing, evaporation, sublimation, boiling and melting are phase changes associated with phase equilibria. A Pressure-Temperature diagram depicting all three phases of matter is called a phase diagram. Discuss transparencies of phase diagrams. Contrast the phase diagrams for water and carbon dioxide (fig.’s 13-18 and 13-19, pp. 404-405). (5 min.) Assessment: Each student will do in class problems 57 and 58 p.415 (5 min) Vapor Pressure: The phase equilibrium between gas and liquid (Liquid + Energy <=> Gas) describes the vaporization process called evaporation that requires vapor pressure. When the vapor pressure equals the system pressure (for open vessels, the system pressure is atmospheric pressure), then the liquid boils. At the boiling point of a pure substance the vapor pressure is called the saturated vapor pressure. In an ideal gas mixture, the total gas mixture pressure is the sum of the partial pressures of the gases that constitute the mixture (Dalton’s Law of Partial Pressures). The ratio of the partial pressure of a particular gas to its saturated vapor pressure at the temperature is defined as the relative humidity. (5 min) Assessment: Each student will do in class problem 60 p.415 (5 min) Homework: Problems 50, 55, 62 and 64 , p. 415 due 2/2/06. Wednesday 2/1/06 Thursday 2/2/06 Review and Assessment Go over the objectives listed below CHAPTER 13 TEST Friday 2/3/06 Objectives: Be able to use temperature to calculate thermal expansion and thermal stresses. Use the ideal gas law to predict gas properties Understand that temperature is proportional to the average translational kinetic energy of the gas molecules. Be able to determine the dew point and relative humidity of humid air from vapor pressure tables of water. Know how to determine the heat of fusion using a calorimeter. Lesson Plans: PHYSICS Monday 2/6/06 Tuesday 2/7/06 Wednesday 2/8/06 Lessons 14.1-14..6 (Heat—> Q) Sensible Heat: Q=mcT; The specific heat capacity, “c” Latent Heat: Q=m; Specific heat of Fusion and Specific heat of Vaporization Analysis of the lab, “Determination of the Molar Heat of Fusion of Ice” Chemical Potential: Methods to determine the heat of combustion Lessons 14.7-14.9 (Heat Transfer: Conduction, Convection and Radiation) Students at the board: solve problems 50, 55, 62 and 64 , p. 415 and problem 22 p. 439 (15 min.) Conduction: The heat transfer rate in Joules per second = Q/t = kA(T/x), where k is the thermal conductivity (J/(s-m-deg C); T= (where is the temperature on the outer surface, deg K, and T1 is the temperature on the inner surface, deg K x = thickness of the conducting body normal to the heat transfer surface in meters and ; A= heat transfer surface area in square meters. .Model example 14-11, p. 430. (5 min) Assessment: : Each student will do in class problem 39 p. 440. (10 min) Convection: Q/t = hA(T), where h is the convection coefficient (J/(s-m2-deg C); T= (where is the temperature on the outer surface, deg K, and T3 is the temperature of the bulk fluid, deg K. (5 min.) Assessment: If the heat transfer coefficient, h, is 0.0814 (J/(s-m2-deg C), for natural convection to air from a vertical wall 1 meter tall x 10 meters wide, what heat loss can be expected by convection when the wall temperature is 20 deg C and the temperature outside is 4 deg C? (5 min.) Radiation: Q/t = fA(), where is the emissivity of the object and = 5.67 x 10-8 (W/(m2-degK4) is the Stefan-Boltzmann constant, and; f is a space factor. Discuss “insolation” and model example 14-15, p. 436. (10 min.) Assessment: Each student will do in class problem 33 p. 440 (10 min.) Homework: Problems 34 and 36 , p. 440. You will solve these problems at the board tomorrow. Lessons 15.1-15.6 (The First and Second Laws of Thermodynamics) Students at the board: solve problems 34 and 36 , p. 440 (5 min.) The First Law of Thermodynamics: U = Q – W, where U is the change in the internal energy of a closed system; Q is the net heat added to the system, and; W is the net work done by the system . Model example 15-1, p. 444. (5 min.) Adiabatic processes are those in which no heat is allowed to enter or leave a system (i.e., Q=0). Therefore, U = -W. Model example 15-5, p. 448 (5 min.) Isobaric processes are carried out at constant pressure, P. For a gas, W=P(V 2-V1) For an ideal gas U=(3/2)nR(T2-T1) = 3/2W, so Q = 5/2W. For a phase change (liquid + energy => gas), Q= m so U = m - P(V2-V1) , where is the specific heat of vaporization. Model example 15-6, p. 448 (5 min.) Isochoric processes are carried out at constant volume, V. Since V=0, there is no work done by the system. (i.e., W=0). Therefore, U = Q . (2 min.) Isothermal processes are carried out at constant temperature, T. For an ideal gas (only!!!!) U=(3/2)nR(T2-T1)=0, since T2 = T1 . Therefore Q = W. Also P 1V1 = nR T1 = P2V2 = nR T2 . .Since the work for an isothermal process between P 1, V1 and P2, V2 is the sum of the work for an isochoric process between P 1,V1 and P2, V1 and an isobaric process between P2,V1 and P2, V2, then W = P2(V2-V1). Model example 15-4 . (5 min.) Assessment: Each student will do in class problems, 2and 7 pp. 471 and 472 (5 min) The Second Law of Thermodynamics: Heat flows naturally from a hot (High temp.) object to a cold (Low temp.) object. Heat engine model: QH-QL=W. (5 min) Efficiency: efficiency = e=W/ QH = (QH-QL)/ QH . Model example 15-8, p. 453. (5 min) Assessment: Each student will do in class problem 12 p.413 (5 min) The Carnot Cycle: See fig. 15-12, p. 454. The maximum possible efficiency of a heat engine is = (TH-TL)/ TH Model example 15-10, p. 455. (5 min.) Assessment: Each student will do in class problems 17 and 19 p.473 (5 min) Refrigerators, Air Conditioners:. COP1 = coefficient of performance for cooling = QL / W. = QL / (QH-QL ). (1 min) Thursday 2/9/06 Heat Pumps: COP2 = coefficient of performance for heating = QH / W. = QH / (QH-QL ) = = W + QL )/ (QH-QL ) = 1+ COP1 (1 min.) Assessment: Each student will do in class problem 29 p.473 (4 min) Homework: Problems 3, 5 and 20 , pp. 471-473. You will solve these problems at the board tomorrow. Lessons: 15.7-15.12 (Entropy) Students at the board: solve Problems 3, 5 and 20 , pp. 471-473 (10 min). The Change in Entropy: S = Q/T > 0 . Model examples 15-12 and 15-13, p. 459. The total entropy of any system plus that of its environment increases as the result of any natural process.(10 min) Assessment: Each student will do in class problem 34 p.473 (5 min) Review: (30 min.) Temperature: K=C+273; F=1.8C + 32 Thermal Expansion: L/Lo = T, where “” is the coefficient of linear expansion (see table 13-1, p. 388 Thermal Stresses: F/A=ET, where “E” is the elastic modulus The Ideal Gas Law: PV=nRT= N(R/NA)T=NkT, where k= =Boltzmann’s constant= 1.38 x 10-23 J/K. Internal energy: U=N(1/2mv2)= 3/2NkT=3/2nRT Latent and Sensible Heat: Q=mcT, where c is the specific heat capacity. Friday 2/10/06 There will be a brief test on chapter 14 and chapter 15 on Monday Lesson Plans: PHYSICS Monday 2/13/06 Tuesday 2/14/06 Wednesday 2/15/06 Review of Chapters 14 and 15 Test on chapter 14 and chapter 15 Lessons 16.1-16.6 (Static Electricity; Coulomb’s Law) The Law of Conservation of Electric Charge: The net amount of electric charge produced in any process is ZERO! Charges are either negative or positive. Unlike electric charges attract; like charges repel. Free electrons in a material make the material a conductor of electricity. Bound electrons make the material an insulator. An object in contact with another positively charged object becomes positively charged due to the transfer of electrons from it to the other object. On the other hand, if the other positively charged object is brought near to, but not in contact, with the object, a charge will be induced in the object due to separation of charge within the object. Charges that are at rest are called “static” charges.(5 min) Assessment: : Each student will use the electroscope, a comb, plastic rulers, glass rods and a grounded conductor to determine the sign of the charge of the objects. Then, each student will answer questions 1 through 5, p.496. (25 min) Coulomb’s Law: F=k(Q1Q2/r2) is completely analogous to Newton’s law of universal gravitational attraction. Here, k = 8.988 x 109 N-m2/C2, where “C” is the coulomb, the SI unit of charge. Q1 and Q2 are the static charges on either of two charged objects at rest separated by a distance “r” significantly greater than the size of the objects. F is the magnitude of force acting at a distance due to these charges. A negative force is an attractive force, while a positive force is repulsive. The magnitude of the charge on one electron = e = 1.602 x 10-19 Coulombs. (5 min.) Assessment Each student will do problems 1, 2 and 3 , p. 497 (5 min.) Forces due to multiple charges: Electrostatic forces are vectors with both magnitude and direction. Model examples 16-3 and 16-4, pp. 484 and 485.(10 min.) Assessment: Each student will do in class problem 11 and 14 p. 497 and 498 (10 min.) Homework: Problems 12 and 17 , p. 498. You will solve these problems at the board tomorrow. Lessons 16.7-16.10 (The Electric Field) Students at the board: solve problems 11 and 14 p. 497 and 498 (5 min.) The Electric Field: E = F/q, where E is the magnitude of the electric field strength (N/C) between two static charges of magnitudes q and Q. “q” is a positive test charge so that the direction of the electric field depends on whether “Q” is positive (therefore repelling “q”) or negative (thereby attracting “q”). Substituting Coulomb’s law for F gives E = k(qQ/r 2)/q = k(Q/r2). Model examples 16-5, 16-6 and 16-7, pp. 487-488. (10 min.) Assessment: Each student will do in class problems, 21, 22 and 23 p. 498 (10 min) Electric Field Strength as a Vector: When dealing with the electric field strength above or below two point charges, resolve the electric field strength into its orthogonal components, then calculate the magnitude from the pythagorean theorem and the direction using trigonometric identities. Model example 16-8, p. 489 (10 min) Assessment: Each student will do in class problems 24, 25 and 27, p. 498 (10 min) Electric Field Lines (Lines of Force) The direction of a field line is the direction of force that a charge, Q, would have when interacting with a positive point charge, q. . Field lines indicate the direction of the electric field; the field points in the direction tangent to the field line at any point. Field lines are drawn so that the magnitude of the electric field, E, is proportional to the number of lines crossing unit area perpendicular to the lines. The closer the lines, the stronger the field. Electric field lines start on positive charges and end on negative charges; and the number starting or ending is proportional to the magnitude of the charge. Field lines are shown in Fig. 16-29, p. 491 (10 min) Assessment: Each student will do in class problem 34 and 38 p.499 (10 min) Thursday 2/16/06 Interact with computer tutorial on electricity (30 min.) Friday 2/17/06 Block Day, no class Energy cannot be created or destroyed, although in many processes energy is transferred to the environment as heat. As a basis for understanding this concept: a. Students know heat flow and work are two forms of energy transfer between systems. b. Students know that the work done by a heat engine that is working in a cycle is the difference between the heat flow into the engine at high temperature and the heat flow out at a lower temperature (first law of thermodynamics) and that this is an example of the law of conservation of energy. c. Students know the internal energy of an object includes the energy of random motion of the object’s atoms and molecules, often referred to as thermal energy. The greater the temperature of the object, the greater the energy of motion of the atoms and molecules that make up the object. d. Students know that most processes tend to decrease the order of a system over time and that energy levels are eventually distributed uniformly. e. Students know that entropy is a quantity that measures the order or disorder of a system and that this quantity is larger for a more disordered system. f.* Students know the statement “Entropy tends to increase” is a law of statistical probability that governs all closed systems (second law of thermodynamics). g.* Students know how to solve problems involving heat flow, work, and efficiency in a heat engine and know that all real engines lose some heat to their surroundings. Objectives: Students know charged particles are sources of electric fields and are subject to the forces of the electric fields from other charges. Students know the force on a charged particle in an electric field is qE, where E is the electric field at the position of the particle and q is the charge of the particle. Students know how to calculate the electric field resulting from a point charge. Students know static electric fields have as their source some arrangement of electric charges. Students know how to apply the concepts of electrical and gravitational potential energy to solve problems involving conservation of energy. Lesson Plans: PHYSICS Monday 2/20/06 Tuesday 2/21/06 Wednesday 2/22/06 Holiday Review Chapter 16 Test on Chapter 16 Lessons 17.1-17.11 (Electric Potential, Capacitance and Storage) The Electrical Potential: Va=(PEa)/q, where Va, the electrical potential in volts (1 volt=J/C), is the electrical potential energy, PEa, per unit charge, q, measured at some point , a,. The “potential difference” , Vab , is the negative of the ratio of the work done by the electric force to move an electric charge from point b to point a, Wba, in an electric field and the charge, q (i.e., Vab = Va – Vb = -Wba/q). Electrical potential is completely analogous to “height” in gravitational potential energy (i.e., PE = mgh). Review Table 17-1, p. 504. The amount of potential energy transformed is proportional to how much charge flows. Model example 7.1, p. 505 (10 min) Assessment: Each student will do in class problems 1, 3, 4,p. 522 (10 min.) Electrical Potential and Electric Field Strength: Since, E = F/q, and Wba=q Vba = Fd, where d is the distance parallel to the field lines between points a and b, then W ba = q Vba = qEd, so Vba = Ed. Hence, E = Vba /d. Model example 17-2. Note that if q = the charge on the electron (1.6 x 10 –19 C) and Vba = 1 volt , then Wba = q Vba = (1.6 x 10 –19 C)(1 V) = 1.6 x 10 –19 J = 1 electron-volt. The electric potential due to a point charge is Vba = Ed = kQ(d/d2) = kQ/d. Model example 17-3. (10 min.) Assessment: Each student will do in class problems 5, 6, 8 and 14 p. 522 (10 min) Thursday 2/23/06 Capacitance and Electrical Storage: The ratio of charge to voltage is the capacitance, C (1 Coulomb/ 1 Volt = 1 Farad ). A capacitor is a device to store electricity that consists of two conducting objects placed near each other but not touching. Without proof (calculus is required) the energy stored in a capacitor is 1/2 x QV =1/2 x CV2 = ½ Q2/C. The capacitance of a parallel plate capacitor is given by C = A/d, where is the permittivity of the material between the plates, A is the surface area of each plate and d is the distance between the plates. The energy density is defined as the energy per volume of gap space = (1//2 x CV2 )/(Ad) =1//2 x (A/d) (Ed)2 )/(Ad) = ½ E2 . Model example 17-9, p. 517 (10 min.) Assessment: Each student will do in class problems 30, 41 and 43 p.524.(10 min) Homework: Problems 31 and 44 , p. 524 due 4/26/05. You will solve these problems at the board tomorrow. Interact with computer tutorial on electricity Measure current and voltage Observe capacitors There will be a test on chapter 17 on Monday Friday 2/24/06 Objectives: Students know how to apply the concepts of electrical and gravitational potential energy to solve problems involving conservation of energy. Electric Potential and Electric Potential Energy—what are they and how are they related? What is Voltage and how does it relate to electric potential? How are the electric potential and the electric field related? The Electron Volt, a unit of energy—how do I calculate it? What is the electric potential around a point charge or point charges? How do I use this to calculate the potential surrounding an Electric Dipole? What is a Capacitor, how is it made, what does it do? How can I use it to Store Energy? Lesson Plans: PHYSICS Monday 2/27/06 Tuesday 2/28/06 Wednesday 3/1/06 Lessons 18.1-18.7 (Electric Current, Resistance and Power) Students at the board: solve problems 31 and 44 , p. 524 (5 min.) Electric Current: Electric current (I) is the change in current with time (Q/t). Convention is that current flows from the positive electrode of the power source to the negative. Current is measured in amperes (A), where 1 A = 1 C/s. (5 min.) Assessment: Each student will do in class problems 1 and 2 p. 551 (5 min) Resistance and Ohm’s Law: An “ohmic device” is one that follows Ohm’s law: V= IR, where R is the resistance. Resistance is measured in ohms (), where 1 = 1 V/A. Model example 18-3, p. 533. Resistance depends on the length of conductor wire (L) as well as its cross-sectional area (A) , as well as to the “resistivity” which is a property of the material. Resistivity is sensitive to temperature however. Model example 18-6, p. 536. (10 min) Assessment: Each student will do in class problems 5 and 8 p. 498 (5 min) Electric Power Power =P =IV =I2R = V2/R for an ohmic device. Model examples 18-7 and 18-8, p. 539 (10 min) Assessment: Each student will do in class problem 23, 24 and 25 p.552 (10 min) Power in Household Circuits: Model example 18-10, p. 541. Discuss fuses and circuit breakers.(5 min) Assessment: Each student will do in class problem 34, p. 552 (5 min) Homework: Problems 30 and 32 p. 552 Lessons 18.8-18.10 (Alternating Current) Students at the board: solve problems 30 and 32 p. 552 (5 min.) Alternating Current: V=Vosin(2ft) describes a sinusoidal voltage typical of an AC power generator. The voltage across an ohmic resistor is V=IR. Therefore, I= V/R = Vosin(2ft/R = Iosin(2ft) (5 min) AC Power in an ohmic resistor: P =IV =I2R =( Io)2sin2(2ft)R. From this the average power is 1/2( Io)2)R=(IRMS)2R. Discuss peak current and voltage vs RMS current and voltage. Model example 18-11, p. 543 (10 min) Assessment: Each student will do in class problems 40 and 41, p. 553. (5 min) Lesson 19.1(Resistors in Series and Parallel) Meet in Room 228 Interact with computer tutorial on electricity (30 min.) Circuit construction by students (30 min.). The current remains the same for resistors in series. The voltage remains the same for resistors in parallel. Model example 19-2 and 19-3 (10 min.) Assessment: Each student will do in class problem 1, 2, 3 and 7, p. 581 (20 min) Homework: : Problems 4 and 5, p. 581 Lab: Building Electrical Circuits Thursday 3/2/06 Friday 3/3/06 Objectives Students know how to predict the voltage or current in simple direct current (DC) electric circuits constructed from batteries, wires, resistors, and capacitors. Students know how to solve problems involving Ohm’s law. Students know any resistive element in a DC circuit dissipates energy, which heats the resistor. Students can calculate the power (rate of energy dissipation) in any resistive circuit element by using the formula Power = IR (potential difference) I (current) = I R. 2 Lesson Plans: PHYSICS Monday 3/6/06 Lessons 19.2-19.4 (EMF and Kirchoff’s Rules) Review: each student will do problem 15, p. 581 at the board. (5 min.) Electromotive Force: EMF (E) is the potential difference between the terminals of a power source when there is no current. When current flows, the terminal voltag, V, is the difference between the EMF and the internal resistance of the power source. Model example 19.7, p. 563 (10 min) Assessment: Each student will do in class problems 18 and 19, p. 582 (10 min.) Tuesday 3/7/06 Wednesday 3/8/06 Homework: Problems 21 and 31 , p. 582 Heads up!!!! TEST ON CHAPTER 19 DUE BLOCK PERIOD. Lessons 19.5-19.7 (Emf’s in series and parallel, Capacitors in circuits and RC circuits) Students at the board: solve problems 21 and 31 , p. 582 (5 min.) Charging a battery: When two power sources (batteries) are placed in series for charging, the positive terminal of the charging battery (higher voltage) is connected to the positive terminal of the battery to be charged (lower voltage) in order to force charge into the the lower voltage battery. See figure 19-14a, p. 568. (5 min.) Assessment: Each student will do in class problem 36 p. 582 (5 min) Capacitors in circuits: See fig. 19-15a and b, p. 569. Model example 19-9, p. 570. (10 min) Assessment: Each student will do in class problems 37, 38, 42 and 49 p. 583 (10 min) RC circuits V = E(1-exp(-t/RC) describes the voltage across a capacitor in a charging RC circuit. Demonstrate this using a breadboard circuit with capacitor and LED and the voltage probe. Discharge of a capacitor, initially at voltage V o is characterized by an exponential decay function, V = Voexp(-t/RC). Demonstrate an oscillating circuit in which the capacitor cycles through charging and discharging.(15 min) Assessment: Each student will do in class problem 50 and 53 p.583 (10 min) Lessons 20.1 and 20.2 (Electric currents and magnetism) Thursday 3/9/06 Friday 3/10/06 Kirchoff’s Ru;es: Junction Rule—at any junction point, the sum of all currents entering the junction must equal the sum of all currents leaving the junction. Loop rule—the sum of the changes in potential around any closed path of a circuit must be zero. Model example 19-8, p. 566. Read box on problem solving, p. 567. 10 min.) Assessment: Each student will do in class problems 24, 28, 30, 33 and 34, p. 582 (30 min) Interact with computer tutorial on electricity (30 min.) A right hand rule—when the thumb points in the direction of the conventional (positive) current in the wire, the right hand curls around the wire in the direction of the magnetic field. (10 min.) Assessment: Each student will verify that the direction of a magnetic field on a horse shoe magnetic is from north pole to south pole (20 min) Homework: : read pp. 589 to 599 Build R-C Circuits Circuit construction by students; investigation with magnetic field probe, iron filings, magnets and current carrying wire (30 min.). One way or another: Resistors in Series and Parallel—what do you know? And if all else fails how do I use Kirchhoff's Rules for more complicated circuits? What is EMF and in what ways does it differ from 'Terminal Voltage'? In what ways can you Connect Batteries and what will happen when you do that? Series and Parallel—this time with Capacitors, and with a twist! What is it? RC (resistance and capacitance)-Circuits—how can they be used to build circuits that do 'this and then that'? ...and what is this and that anyway? Measurement is always the critical element. How do Ammeters and Voltmeters work? Lesson Plans: PHYSICS Monday 3/13/06 Tuesday 3/14/06 Wednesday 3/17/06 Thursday 3/18/06 Lessons 20.3 (Definition of the Magnetic Field) Force on an electric current in a magnetic field: Our objective is to derive the equation for the magnetic field strength experimentally. We will construct a circuit consisting of variety of resistors in series and parallel configurations, a 9-V battery, an ammeter and a long loop of wire. We will suspend the loop circuit on the force balance. The loop will be suspended between the north and south poles of a magnet so that it crosses the magnetic field perpendicularly. We will use the magnetic field sensor to determine the value of the magnetic field strength, B, in milliTesla (mT). The Tesla is the SI unit of magnetic field strength. To determine the relationship, current will be varied systematically. If time permits, the loop angle will also be systematically varied also. (50 min) Assessment: Each student will do in class problems 1 and 2, p. 615 (10 min.) Homework: Problems 3 and 4 , p. 616 Lesson 20.5 (Magnetic Field due to a Straight wire) Students at the board: solve problems 3 and 4 , p. 616 (10 min.) Permeability of Free Space: Our objective is to determine the effect of distance from a current carrying wire on the magnetic field strength. Using the magnetic field sensor, we will plot B vs. r at a constant current flow. The permeability of free space will be calculated from the data.(40 min.) Assessment: Each student will do in class problem 19 and 20 p. 617(10 min) Homework: Problems 21 and 22 , p. 617 Lessons 20.4, 20.6 and 20.8 (Magnetic Field Effects) Students at the board: solve problems 21 and 22 , p. 617 (10 min.) Force on an electric charge moving in a magnetic field: F=BIlsin applies to a confined current. The product of a free electric charge, q, and its velocity, v, is qv. This is equivalent to IL. So, F=qvBsin. (5min) Assessment: Each student will do in class problem 5 and 6 p. 616(10 min) Solenoids: We will determine the magnetic field through a coil of “n” loops per meter carrying a current,I, experimentally. (25 min) Assessment: Each student will do in class problem 36 p. 618 (10 min) Homework: Read pp. 622 to 627 of Giancoli for tomorrow. Lessons 21.1 and 21.2 (Induction) Inducing an electric current by a changing magnetic field: We will measure the current induced in a coil of wire using a magnet, field sensor, a coil of wire of predetermined resistance and the ammeter. The objective is to determine Faraday’s Law of Induction experimentally (60 min.) Assessment: There will be a class discussion about the observations and conclusions made during this experiment. (30 min) Homework: : read pp. 627 to 633 of Giancoli for Monday Friday 3/19/06 Students know magnetic materials and electric currents (moving electric charges) are sources of magnetic fields and are subject to forces arising from the magnetic fields of other sources. Students know how to determine the direction of a magnetic field produced by a current flowing in a straight wire or in a coil. Students know changing magnetic fields produce electric fields, thereby inducing currents in nearby conductors. Students know plasmas, the fourth state of matter, contain ions or free electrons or both and conduct electricity. Students know electric and magnetic fields contain energy and act as vector force fields. Students know the force on a charged particle in an electric field is qE, where E is the electric field at the position of the particle and q is the charge of the particle. Students know how to calculate the electric field resulting from a point charge. Students know static electric fields have as their source some arrangement of electric charges. Students know the magnitude of the force on a moving particle (with charge q) in a magnetic field is qvB sin(a), where a is the angle between v and B (v and B are the magnitudes of vectors v and B, respectively), and students use the right-hand rule to find the direction of this force. Students know how to apply the concepts of electrical and gravitational potential energy to solve problems involving conservation of energy. Lesson Plans: PHYSICS Monday 3/20/06 Tuesday 3/21/06 Wednesday 3/22/06 Thursday 3/23/06 Lessons 21.1-21.3 Induced EMF, Faraday’s Laws, EMF Induced in a Moving Conductor) Unit 20 exam due today. Induced EMF: Read pp. 622 & 623. (10 min) Assessment: Each student will do in class problems 1 and 4, p. 654 (10 min.) Faraday’s Law of Inductance: Read pp. 624 & 627. (10 min) Assessment: Each student will do in class problems 5 and 6, p. 654 (10 min.) EMF Induced in a Moving Conductor: Read pp. 627 & 628. (10 min) Assessment: Each student will do in class problem 13, p. 654 (10 min.) Homework: Problem 8, p. 654 Lessons 21.5-21.7 (Electric Generators, Counter EMF and Torque, Transformers) Solve problem 8 , p. 654 (10 min.) Electric Generators: Read pp. 629-631. (10 min) Assessment: Each student will do in class problems 19 and 20, p. 655 (10 min.) Counter EMF and Torque: Single silent reading (SSR) pp. 631-633. (10 min) Assessment: Each student will do in class problems 24 and 25, p. 655 (10 min.) Transformers: Read pp. 633-637. (10 min) Assessment: Each student will do in class problems 30 and 31, p. 655 (10 min.) Homework: Problem 32 , p. 656 Lessons 21.8-21.11 (Inductance, Energy Stored in a Magnetic Field, LR Circuit) Solve problem 32 , p. 656 (10 min.) Inductance: Read pp. 637-640. (5 min) Assessment: Each student will do in class problems 42 and 43, p. 656 (10 min.) Energy Stored in a Magnetic Field: Read pp. 640-641. (5 min) Assessment: Each student will do in class problems 54 and 55, p. 657 (10 min.) LR Circuit: Read pp. 641-642. (5 min) Assessment: Each student will do in class problems 57 and 58, p. 657 (10 min.) Homework: Problem 56 , p. 657 Lessons 21.12 - 21.15 (AC Circuits and Impedance, LRC Circuits, Resonance in AC Circuits) Solve problem 56 , p. 657 (10 min.) AC Circuits and Impedance: Read pp. 642-646. (10 min) Assessment: Each student will do in class problems 61 and 62, p. 657 (10 min.) LRC Circuits: Read pp. 646-649. (10 min) Assessment: Each student will do in class problems 70 and 71, p. 657 (10 min.) Resonance in AC Circuits: Read pp. 649-651. (10 min) Assessment: Each student will do in class problems 81 and 85, p. 658 (10 min.) Homework: : read pp. 660 to 669 Friday 3/24/06 Objectives g. Students know how to determine the direction of a magnetic field produced by a current flowing in a straight wire or in a coil. h. Students know changing magnetic fields produce electric fields, thereby inducing currents in nearby conductors. j.* Students know electric and magnetic fields contain energy and act as vector force fields. Lesson Plans: PHYSICS Monday 3/27/06 Lessons 22.1-22.4 (Electromagnetic Waves: Gauss’s Law; Maxwell’s Equations, and: the Production of Electromagnetic Waves) Gauss’s Law: E = Q/o Q = net enclosed charge of a closed surface o = permittivity of free space = 8.85 x 10-12 C2/(N-m2) E = (Ei(Ai)cosiTotal Electric Flux through a closed surface Ei = electric field strength for a tiny area Ai I = the angle between the electric-field direction and a line drawn perpendicular to the area, Ai. Assessment: A thin spherical shell of radius ro possesses a total net charge Q that is uniformly distributed on it. Determine the electric field at points (a) outside the shell and (b) inside the shell. (example D-1 in appendix D). Maxwell’s Equations: (see handout) In vacuum, without charges or currents The vacuum is a linear, homogeneous, isotropic, dispersionless medium, and the proportionality constants in the vacuum are denoted by ε0 and μ0 (neglecting very slight nonlinearities due to quantum effects). 0 = permeability of free space = 4 x 10-7 T-m/A Since there is no current or electric charge present in the vacuum, we obtain the Maxwell's equations in free space: These equations have a simple solution in terms of travelling sinusoidal plane waves, with the electric and magnetic field directions orthogonal to one another and the direction of travel, and with the two fields in phase, travelling at the speed Maxwell discovered that this quantity c is simply the speed of light in vacuum, and thus that light is a form of electromagnetic radiation. Production of Electromagnetic waves: If a changing magnetic field produces an electric field, that electric field is itself changing. The changing electric field, however, produces a magnetic field which itself is changing too!! The Maxwell equations therefore show that “waves” of electric fields and magnetic fields can propogate (travel) through space. Tuesday 3/28/06 Assessment: Problems 1-4 and 7-9 p. 680 (E=B/c) Lessons 22.5-22.7 (Light as an Electromagnetic Wave and Energy in EM Waves) c = 3.00 x 108 m/s Michelson Experiment: How can an eight-sided mirror rotating at angular velocity determine the speed of light? See figure 22-12. Assessment: Problem 19, p. 681 Energy in EM waves: u = energy density u = (1/2)oE2 + (1/2)B2/o = oE2 = B2/o , J/m3 Wednesday 3/29/06 The Poynting Vector: S = U/(At) = energy crossing area A per time t U =uV = energy in volume V S = ocE2 = cB2/o, J/(s-m2) = W/m2 Assessment: Example 22-3 p. 675 and Problems 11, 13, 22-25 p. 681 Lessons 22-8 (Radio and TV) Amplification of Audio Signals and Radio Frequency Signals: When a weak signal (i.e., small current, IB) controls a much larger current (IC), the weak signal can be amplified. Transistors allow weak signals to be amplified. Carrier Frequency-RF Oscillators: These are simply LRC circuits that resonate at a particular frequency. AM radio has carrier frequencies between 530 kHz to 1600 kHz and FM radio has frequencies between 88 MHz and 108 MHz. TV stations have carrier frequencies between 54 MHz and 88 MHz (channels 2 through 6); 174 MHz and 216 MHz (channels 7 through 13), and; 470 MHz through 890 MHz (UHF). Assessment: Problems 30 and 31, p. 681 Mixers: Mixing is done in two ways. Amplitude Modulation (AM) adds audio signal to carrier signal such that the amplitude of the carrier signals follows the audio signal. Frequency Modulation (FM) varies the frequency of the carrier signal in proportion to the audio signal. (FM is a special case of Phase Modulation (PM)) Antennae: In its simplest form, an antenna is just a length of wire. Its function being to convert electromagnetic energy into voltage for reception, or to transduce a varying voltage into electromagnetic energy for transmission. The whip antenna, when positioned vertically will transmit and receive in all directions (omnidirectional). The dipole antenna is made from two lengths of straight wire and can be arranged horizontally or vertically. Depending upon its orientation, a transmitted wave will either be horizontally or vertically polarized. When using a dipole it is important to make sure that both receiving and transmitting antenna have the same orientation. Assessment: Problems 34 and 49, pp. 681-682 Thursday 3/30/06 Lab: Spectroscopy Friday 3/31/06 Objectives Students know the properties of transistors and the role of transistors in electric circuits. Changing magnetic fields produce electric fields. Do Changing Electric Fields Produce Magnetic Fields? Maxwell's Equation's what are they? I know what current is but what is Displacement Current and how do I use it? If Electromagnetic Waves are composites of changing electric and magnetic fields, how are they generated? I know that all Electromagnetic Waves travel at the Speed of Light. How is it calculated? The Electromagnetic Spectrum what are...Radio Waves? Microwaves? IR radiation? UV? X-rays? Gamma Rays—how are they the same and how do they differ? The Sun warms the Earth. How do Electromagnetic Waves carry Energy? Radio and Television—I use them, how do they work? Lesson Plans: PHYSICS Monday 4/3/06 Tuesday 4/4/06 Lessons 23.1-23-3 (Light: Geometric Optics—The Ray Model and Reflection from Plane and Spherical Mirrors) The Ray Model of Light: Light travels in straight lines. Light from distant objects has parallel rays. Light from near objects has divergent rays. Reflection from a Plane Mirror: The angle of incidence, i, is the angle between the incident light ray and the normal to the reflecting surface. The angle of reflection, r, is the angle between the reflected ray of law and the normal to the reflecting surface. The Law of Reflection is the angle of incidence is equal to the angle of reflection (i = r ). The consequence of the Law of Reflection for a plane mirror is that the image distance, d i, (distance from mirror to image) is equal to the object distance, do, (distance from object to mirror). When light rays do not actually pass through the image, the image is termed a “virtual image”. Reflected light from a plane mirror produces virtual images. Assessment: Problems 1, 3 and 5 p. 717 Reflection from Spherical Mirrors: Concave mirrors have reflecting surface on the inside surface of a sphere. Convex mirrors have reflecting surface on the outside of a sphere. For only those spherical concave or convex mirrors with large radius of curvature, r, compared with its width, light rays from distant objects (i.e., parallel rays) will, approximately, meet at a focal point with focal length, f = r/2. (assume both focal length, f, and r are negative for a convex mirror). For near objects of vertical height ho, located at object distance do from the mirror, the mirror equation applies: 1/ do + 1/ di = 1/f. The lateral magnification “m” = h i/ ho = -di/do . The minus sign tells us the image is inverted (upside down). However, when m is positive, the image is not inverted (right side up). When the image distance di is positive, then the image is on the same side of the mirror as the object. This is a real image. When the image distance is negative, the image is “behind” the mirror. This image is a virtual image. Shaving mirrors are concave because, when the object is closer than the focal point, the image is virtual, upright and magnified. Rearview car mirrors are convex because distant objects are virtual, upright and smaller than the object. Assessment: Problems 9, 10 and 13 p. 718 The chapter 23 exam is due next block period. Lessons 23.4 –23.8 (Light: Index of Refraction, Snell’s Law, Total Internal Reflection ,Thin Lenses and the Lens Equation) Index of Refraction: Light travels more slowly in matter than in a vacuum due to the absorption and reemission of light by atoms and molecules of the material. If “v” is the velocity of light in a material, than its index of refraction n = c/v, where c = 3.00 x 10 8 m/s. Note that the index of refraction depends on the wavelength of light! Assessment: Problems 26 and 27 p. 718 Snell’s Law: The bending of light at a material interface is called refraction. If “1” denotes the incident ray of light, and “2” denotes the refracted ray, then Snells law (the Law of Refraction) is: n1sin1 = n2sin2. Assessment: Problems 30, 31 and 34 p. 719 Total Internal Reflection: When 2 = 90 , the incident angle is called the “critical” angle. At this angle, and for all incident angles greater than the critical angle, all of the light is reflected. This only can happen when n2 < n1. Assessment: Problem 40, 41 and 46 pp.719-720 Thin Lenses: Any lens that is thicker in the center than at the edge will make parallel incident rays converge to a point, and is called a converging lens. Any lens thinner in the center than at the edge will make parallel rays diverge and is called a diverging lens. The “power” of a lens P = 1/f (m-1). Ray tracing is a three-step method to determine where the image of a point on the object will be formed. Refer to figures 23-34 and 23-36. Assessment: Problems 48, 50 and 52 Lens Equation: For both converging and diverging lenses, : 1/ do + 1/ di = 1/f. For a diverging lens, f is negative (as r is negative). When the do is on the incident side of the lens, it is positive. When di is on the incident side of the lens it is negative. Wednesday 4/5/06 Thursday 4/6/06 Assessment: Problems 54 and 57 p. 720 Lessons 23.9 – 23.11 (Light: Problem Solving for Lenses, Combinations of Lenses and the Lensmaker’s Equation) Problem Solving for Lenses: Do examples 23-11, 23-12 and 23-13 Assessment: Problems 60 and 62 p. 720 Combinations of Lenses: In general, the image from the first lens is the object of the second lens. Do examples 23-14 and 23-15 Assessment: Problems 64 and 66 p. 721 The Lensmaker’s Equation: 1/f = (n-1)(1/R1 + 1/R2) for a lens with radii of curvature for the two surfaces of R1 and R2. Lens grinders can be set to grind glass at the desired radius of curvature. “n” is the index of refraction of the glass (or other material) used in the lens. The radius of curvature is positive if the lens surface is convex and negative if the surface is concave.Do example 23-16. Assessment: Problem 68, 69 and 71 p. 721 Lab: Static Charge Friday 4/7/06 I have heard of Light Rays. What is a 'ray' anyway? ...and when is light a ray and when is it not? "Mirror, mirror..." how do you show me what I see? The big mirrors I see in stores, why do they have Curved Mirrored Surfaces? How come I see all the people in the store in them? What different kinds of curved mirrors are there? What is a focal point? What type of images are there? How can I use light rays to predict where images are formed by curved mirrors? What equations can I use to solve these problems? What is a Index of Refraction and what does it tell me about the Speed of Light? How can I use Snell's Law to predict what light does as it goes from one material to another? I have heard of Total Internal Reflection. How does that work? o How is it utilized in Binoculars? o I have seen an advertisement for 'genuine non-prismatic binoculars'. Should I buy them? o With all the talk about the Internet, I have heard lots about Fiber Optic Networks. What is Fiber Optics anyway? o What is a Lens and what are the different types of Lenses? o How can I use Ray Diagrams to find the image formed by a lens? o In what way are these diagrams different from those I used for mirrors? o What Lens Equations are available for solving problems? ...and how can I use them? What is the Lensmaker's Equation and how is it used? Lesson Plans: PHYSICS Monday Lessons 24.1 – 24.3 (Light: Wave Nature—Huygen’s Principle, Diffraction, Refraction and Double-Slit 4/17/06 Interference) Huygen’s Principle: Every point on a wave front can be considered as a source of tiny wavelets that spread out in the forward direction at the speed of the wave itself. The new wave front is the envelope of all the wave fronts—that is, the tangent to all of them. Diffraction is the bending of waves behind obstacles consistent with Huygen’s Principles. Assessment: Demonstrate that diffraction is consistent with Huygen’s Principle using water waves. Using compasses, have students draw expected wave patterns when parallel wavefronts are interrupted by regular shapes of size comparable to the wave length. Check predictions with water waves. Also, problem 1, p. 752. Refraction: According to Huygen’s Principle, the wavelength of light changes at a material interface because the velocity of light is different in the two materials. The frequency remains the same. A light “ray” is always perpendicular to a wave front (because it is a radius and the wave front is tangent to the circles). Assessment: See figures 24-3. What happens when a wave front is curved? Will the light “ray” be straight or curved? See the text on highway mirages. Interference-Double Slit: Discuss constructive and destructive interference with respect to the double slit “apparatus” . Derive that constructive interference occurs when dsin = mwhere m = 0,1,2 and destructive interference occurs when dsin = (m+1/2) Assessment: Problems 2-6 p. 752 The chapter 24 exam is due next block period. Tuesday Lessons 24.4 –24.7 (Dispersion, Single-slit and Multiple Slit Interference, and the Spectrometer ) 4/18/06 Dispersion: The refractive index of materials depends on both the material and the wavelength of the incident light because transmitted light is a result of re-emission by the electrons of the material. The time required to re-emit is proportional to the energy in the incident light (recall that E = h = hc/). Assessment: Problems 13 and 15 p. 753 Interference-Single Slit: For a slit of width “D”, destructive interference occurs when Dsin = m where m = 1,2,3 and constructive interference occurs when Dsin = (m+1/2) See figure 24-19. Assessment: Problems 18, 19 and 20 p. 753 Diffraction Grating—Multiple Slit Interference: Demonstrate transmission gratings and reflection gratings. Note that constructive interference and destructive interference are the same as for double-slit interference with the exception that maxima and minima are much sharper due to the multiplicity of slits. Model examples 24-6 and 24-7. Assessment: Problem 26 -28 p.754 The Spectrometer: Demonstrate how to use the spectrometer for Neon and CO2 gas arcs. Show the difference between line spectrums and continuous spectrums. Assessment: Problems 34 and 35 p. 754 Wednesday Lessons 24.8-24.11 (Thin-film Interference) 4/19/06 Thin Film Interfence: If “t” is the thin film thickness, then light will that reflects from the upper interface will interfere with light reflected from the lower interface because light that passes into the thin film travels further than light that does not. When the path length through the film is an integral multiple of the wavelength in the film, interference is constructive for that wavelength. When the path length is ½ wavelength off, interference is destructive. Assessment: Problems 38-40 p. 754 Phase Change Upon Reflection: At an interface between two media, if n1 > n2 , the reflected beam of light is 180 degrees (i.e., ½ ) out of phase with the incident light. If n 1<n2, then the reflected beam is in phase with the incident beam. Model example 24-8. Assessment: Problems 42-44 p. 754 Interferometry: By introducing a change in optical path length between an incident beam and a reflected beam, then adding both beams together, interference results. Many devices are based on this principle in order to measure very small lengths. The resolution of an interferometer is /4. Assessment: Problems 49-51 p. 754 Polarization: Plane polarization describes polarization at right angles whereby only that component of the electromagnetic vector in the plane of polarization is allowed to be passed through the polarizer. Therefore, a polarizer reduces the intensity of unpolarized incident light (Io) by a factor of ½. Attenuation of the intensity of an electric field is given by I/Io = Thursday 4/20/06 Friday 4/21/06 cos2 Assessment: Problems 54-56 p.755 Lab: Diffraction and Interference Lesson Plans: PHYSICS Monday Lesson Plan 25.1-25-2 (Optical Instruments: Cameras and Glasses) 4/24/06 Cameras: f-stop, depth of field, how to make a telephoto lens and a wide-angle lens Glasses: How glasses correct near-sightedness and far-sightedness Tuesday 4/25/06 Wednesday 4/26/06 Thursday 4/27/06 Friday 4/28/06 Lesson Plan 25.3-25.5 (magnifying glass, telescope, binoculars and microscope) Magnifying Glass Refracting (astronomical) telescope Reflecting telescope Terrestrial telescope Binoculars Microscopes Lesson Plan 25.7-25.9 and 25.11-25.12 (Resolution of two images, resolving power,, numerical aperture, useful magnification and x-ray diffraction) Resolution for telescopes, microscopes and the eye How can X-rays tell us about the structure of matter? What is a CAT Scan Lab: Determine the accommodation, near point and far point of a fellow student. Also, test that student for astigmatism. Finally, determine the focal point and type of lens that allows a farsighted eye to read a newspaper at 25 cm distance and a nearsighted eye to see distant objects clearly and to read a newspaper at a calculated near point. Lesson Plans: PHYSICS Monday 5/1/06 Tuesday 5/2/06 Lesson Plan 26.1-26.2 (Special Theory of Relativity: Galilean-Newtonian Relativity and the MichelsonMorley Experiment) Inertial and Non-Inertial Reference Frames: Non-accelerating reference frames are inertial reference frames. Accelerating or rotating reference frames are non-inertial reference frames. Assessment: Questions 1-3 p. 818 The Relativity Principle: The laws of physics are the same in all inertial reference frames Assessment: A coin is dropped from a height of 3 m in a car traveling at 30 m/s. Determine (a) the time it takes for the coin to reach the floor of the car as measured in the car; (b) the position in the car that the coin will be at when it reaches the floor; (c) the time it takes for the coin to reach the floor as measured by a stationary observer outside the car, and; (d) the position in the framework of the stationary observer that the coin will be at when the coin reaches the floor. See Fig. 26-2 Un-provable Assumptions of Galilean-Newtonian Relativity: Time, the length of objects, the mass of objects and forces are “ASSUMED” to be the same in all reference frames. The position of an object and its velocity are different when specified in different reference frames. Acceleration (the change in velocity with time) is the same in all reference frames. All inertial reference frames are equivalent for the description of mechanical phenomena. Assessment: Question 4 p. 818 The Speed of Light: c = 3.00 x 108 m/s according to the Maxwell Equations (see chapter 22). In what reference frame does light travel at this speed? Would the speed of light be different if it were measured in different directions? Assessment: Refer to figure 26-3. Calculate the round trip times, t1 and t2, required for the same distance “l” for boat 1 and boat 2 traveling at speed “c” perpendicularly and parallel to a downstream current of velocity “v”. The Michelson-Morley Experiment: Model the derivation of the interference fringe shift expected in a Michelson interferometer as a result of the speed of the earth relative to the sun. Assessment: How does the Lorentz contraction (x’/x = (1-v2/c2)0.5 ) explain the “null result” of the Michelson-Morley experiment? Lesson Plan 26.3-26.7 (Special Theory of Relativity: Postulates, Galilean and Lorentz Transformations, Length Contraction and Time Dilation) The First Postulate of Relativity is the Relativity Principle: The laws of physics are the same in all inertial reference frames The Second Postulate of Relativity is the Constancy of the Speed of Light: Light propagates through empty space with a definite speed (c = 3.00 x 10 8 m/s) independent of the speed of the source or observer. Galilean Transformations: If (4-dimensional) time-space “S”=S(x,y,z,t) and S’=S’(x’,y’,z’,t’), where S’ is traveling at velocity “v” in the “x” direction with respect to S, then the Galilean transformation equations are: x = x’ + vt’ x’ = x –vt y = y’ y’ = y z = z’ z’ = z t =t’ t’ = t Suppose a point “P” in S’ is moving with velocity components u x’, uy’ and uz’ such that: ux’ = (x2’ – x1’)/(t2’-t1’) =x’/t’ The velocity of “P” as seen from S will have components ux, uy and uz such that: ux = (x2 – x1)/(t2-t1) =x/t =[ (x2’ + vt2’) – (x1’ + vt1’) ] /(t2’-t1’) ux =[ (x2’ - x1’) +v (t2’- t1’) ] /(t2’-t1’) = =x’/t’ + v = ux’ + v uy = uy’ uz = uz’ Lorentz Transformations: x = (x’ + vt’) x’ = (x –vt) y = y’ y’ = y z = z’ z’ = z t = (t’+vx’/c2) t’ = (t-vx/c2) Because: IFFI (if and only if) light travels at the same speed “c” in both S and S’, then: x = ct x’ = ct’ ct = (ct’ + vt’) ct’ = (ct –vt) ct = (c + v)t’ ct’ = (c –v)t Wednesday 5/3/06 Thursday 5/4/06 Solving for gives: = 1/(1-v2/c2)0.5 Since: x’ = (x –vt) = ((x’ + vt’) –vt) Then: t = (t’+vx’/c2) = (1-v2/c2)-0.5 (t’+vx’/c2) Also: ux =x/t = (x’ + vt’)/(t’+vx’/c2)] = (x’/ t’ + v)/[1 + (v/c2) (x’/ t’)] ux = [ux’ + v]/[1 + (v/c2) ux’] And: uy = uy’(1-v2/c2)0.5/[1 + (v/c2) ux’] uz = uz’(1-v2/c2)0.5/[1 + (v/c2) ux’] When v<<<c, these equations reduce to the Galilean transformation equations. This is called the correspondence principle. Assessment: Problems 1,2, 9 and 10 p. 819 Length Contraction and Time Dilation: Since t’= 0 when measuring a length of x’x = (x’ + vt’)] = x’ = x’ /(1-v2/c2)0.5 Since x’ = 0 for a time interval of t’t = (t’+vx’/c2) = t’ = t’ /(1-v2/c2)0.5 Lesson Plan 26.8-26.10 (Momentum, Mass and Energy) Mass: If m = the rest mass when v=0, then relativistic mass in the direction of motion = m’ = m = m/(1-v2/c2)0.5 Momentum: The momentum of a particle with relativistic mass m’ = p’ = m’v = mv/(1-v2/c2)0.5, where “v” is, as before, the motion of inertial frame S’ in the x direction as observed in the S inertial frame. Assessment: Problems 13-15 p. 820 Kinetic Energy: The binomial expansion of 1/(1-v2/c2)0.5 ~ 1 + ½ v2/c2 when v<<<c. Einstein showed that the kinetic energy =KE =m’c2-mc2 = m/(1-v2/c2)0.5c2-mc2 ~ m(1 + ½ v2/c2)c2-mc2 = ½ mv2 Assessment: Problems 18, 19, 20, p. 820 Rest Energy and Total Energy: The rest energy = mc2. The Total Energy is the sum of the rest energy and the kinetic energy = E = mc2 + (m’c2-mc2) = m’c2, assuming no potential energy. The energy released in a chemical or nuclear reaction, E, results in a mass defect, m’: E = (m’)c2 Assessment: Model the calculation of the energy released from a nuclear fission reaction. Lesson Plan 26.11-26.12 (Relativistic Addition of Velocities and the Impact of Special Relativity) Addition of Velocities: When ux’ and v are in the same direction, ux = [ux’ + v]/[1 + (v/c2) ux’], as derived earlier. When ux’ and v are in opposite directions, ux = [-ux’ + v]/[1 - (v/c2) ux’]. Assessment: Problems 45-48, p.821 Impact of Special Relativity: Read chapters in the selected books and discuss the impact on the politics of warfare; scientific and technological changes; how we view the universe. Friday 5/5/06 What is A Principle of Relativity? How does Special Relativity differ from Classical Relativity? What was the Ether? How did the Michelson Morley experiment dispense with it? What are the basic postulates of Special Relativity? What’s so special about Inertial Observers? What, if any, are the consequences of the statement, “All motion is Relative Motion”? What is meant by the Failure of Simultaneity? What is meant by Time Dilation? What is meant by Lorentz Contraction? How do Relativistic Momentum and Relativistic Energy differ from classical momentum and energy? What is the significance of the equation E = mc2? If all observers see the samespeed for the speed of light no matter what their relative velocities, how do you add velocities? Lesson Plans: PHYSICS Monday Lesson Plan 27.1-27.2 (Quantum Theory and Models of the Atom: The Electron and Black-Body 5/8/06 Radiation) The Effect of a Magnetic Field on a Beam of Electrons: When electrons are produced in a “discharge tube” by applying a high voltage between a “cathode” (negative plate) and an “anode” (positive plate), the electrons will leave the cathode and travel towards the anode with velocity “v”. If only a magnetic field is present of strength “B”, the electrons are deflected. The force exerted on the electrons is F = evB where “e” is the charge. The electrons follow a curved path of radius “r”. The centripetal force (mv2/r) equals the force due to the magnetic field (evB) so, evB = mv2/r. Hence, e/m =v/(Br) The effect of an Electric Field on a Beam of Electrons: If an electric field of strength “E” is applied to the beam of electrons (using capacitance plates through which the electrons move), the force exerted on the electrons is F= eE. The electrons follow a curved path of radius “r”. The centripetal force (mv2/r) equals the force due to the electric field (eE) so, eE = mv2/r. Hence, e/m =v2/(Er) Determination of the Velocity of an Electron: If the electric field is applied perpendicularly to the magnetic field, the deflection of the electron beam due to the electric field will be in a direction opposite to that of the magnetic field. When the deflection of the beam is eliminated by adjusting the values of “E” and “B”, then, e/m =v2/(Er) = v/(Br) v = E/B and e/m = E/(B2r) = 1.76 x 1011 C/kg Assessment: Problems1-2 p. 854. Draw an external circuit for the cathode ray tube of fig. 272 Determination of the Charge on an Electron: In Millikan’s oil drop experiment, an oil drop falls through air. Fluid dynamics shows that the viscous force, F, on a spherical object of radius, r, in a fluid of viscosity, , is F = 6rvT, where vT is the “terminal velocity”. This viscous force equals the buoyant force on the object due to gravity, (m-mf)g, where mf is the mass of fluid displaced by the volume of the object. Hence, for a spherical object: F = 6rvT = (m-mf)g = 4/3 r3(-f)g Which gives: r = ((18/4)vT/((-f)g))0.5 When the oil drop acquires a static charge, q, and then is admitted between the plates of a capacitor of electric field strength, E, then the force due to the electric field, qE, will oppose the buoyant force on the charged particle, 4/3 r3(-f)g. Hence: qE = 4/3 r3(-f)g = 4/3 ((18/4)vT/((-f)g))0.5)3(-f)g = 18 3vT3/((2(-f)g))0.5 Assessment: So, q can be determined from the oil drop experiment. What evidence can be gathered from the Millikan apparatus to prove that q is an integral multiple of e = 1.6 x 10 -19 C? Note that: the mass of an electron = e/(e/m) = 1.6 x 10-19 C/(1.76 x 1011 C/kg) = 9.11 x 10-31 kg. Black-Body Radiation: Radiation is a form of heat transfer such that power = energy/time = Q/t = fA(), where is the emissivity of the object and = 5.67 x 10-8 (W/(m2degK4) is the Stefan-Boltzmann constant, and; f is a space factor. The intensity of radiation for a spherical wave of surface area A = power/area = f ()= I. When = 0 °K, and f =1.0, then I = T4 where = 5.7 x 10-5 erg/(cm2-sec- °K4). Wien’s law relates the wavelength of radiated energy at the peak intensity (p ) for a given temperature (T) as follows: pT = 2.9 x 10-3 m- °K Assessment: Problems 5-7, p. 854. How does Wien’s Law relate to the Kinetic Theory? Tune in to tomorrow’s lesson. Tuesday Lesson Plan 27.3-27.7 (Photons and Photon Interactions) 5/9/06 Planck’s Quantum Hypothesis: E= nh where n is an iteger (1,2,3,….), h = Planck’s constant = 6.626 x 10-34 J-s and (nu) is the frequency of an oscillator. E is the energy of the oscillator. Since the total energy of the oscillator is the sum of the kinetic energy and the rest energy of the oscillator (recall from special relativity that E = mc2 + (m’c2-mc2) = m’c2) and that ,when v<<<c, Einstein showed that the kinetic energy =KE =m’c2-mc2 = m/(1-v2/c2)0.5c2mc2 ~ m(1 + ½ v2/c2)c2-mc2 = ½ mv2) so E= nhmc2 + ½ mv2. The frequency of radiation is therefore a function of the velocity (v) of the particle. The distribution of velocities for “photons” at any temperature “T” is Maxwellian according to the kinetic theory (i.e., f(v) = (m/(2kT))3/2exp(-(mv2/(2kT)) ) so the distribution of the Intensity of radiation emitted from a black body will also be Maxwellian with respect to frequency or wavelength. Photons: E= h = m’c2 = mc2m/(1-v2/c2)0.5)c2 = (p’2c2+m2c4)0.5. For a photon, v=c, hence m=0. Therefore, p’ =h /c = h/. Assessment: Problem 27, p. 854 The Photoelectric effect: E = h = 1/2mv2 + W v =(2 (h-W)/m)0.5 where “W” is the “work-function” of the surface. All of the energy in the photon is transferred to the electron emitted from the atom at velocity v. The photon is annihilated. ( 1 eV = 1.602 x 10 -19 J) Assessment: Problems 12-16 p. 854. Compare the wave theory and the photon theory with respect to the photoelectric effect Scattering of Light (The Compton Effect): Let a photon of frequency scatter off a stationary electron e of rest mass me at an angle . In the collision, it transfers some energy to the electron (Ee - Ee’ ) and emerges at a shifted frequency ’ as ’. Conservation of momentum vectors gives: Where: So: p = p' + pe |pe2| = |(p - p' )2| = pp'p p'cosby the law of cosines p = h /c p' = h' /c |pe2| = (h /c )(h' /c)(h /c ) (h' /c )cos Conservation of energy scalars gives: Where: So: Giving: So: E + Ee = E’ +Ee’ E = h Ee = mec2 E' = h' Ee’ = (|pe2|c2+me2c4)0.5 h+ mec2 = h' +(|pe2|c2+me2c4)0.5 2 2 2 4 2 |pe2| = ((h h'+ mec ) - me c )/ c (h /c )(h' /c)(h /c ) (h' /c )cos((h h'+ mec2 )2- me2c4)/ c2 h’(1-cos) = mec2(-’) Or: hc2/’(1-cos) = mec3(1/-1/’) Therefore: 'h (mec))(1-cos) Giving: Assessment: Problems 31-33, p. 855. Also problem 35, but use relativistic formulas. Photon Absorption: When photons transfer all of their energy to an electron in an absorbing material , the electron may be knocked out of the atom (the photoelectric effect) or may gain energy and go to a higher energy state in the electron configuration around the atom. The photon is annihilated during the single-event transfer of photon energy. If I0 is the intensity of the incident beam and A the absorbed fraction of photons in layers of equal absorbance of the absorbing material then the total absorbance T A of n layers is: TA = A + A(1-A) + A(1-A)2 +…+ A(1-A)(n-1) = In/I0 ~exp(-t) where is the absorption coefficient and t is the total thickness of the n layers. 0 e , where E= h Pair Production: 0 e + -1 +1 electron and positron. Assessment: Problems 26, 28, 29 and 30, p. 855 See Tuesday mec2 for a stationary Wednesday 5/10/06 Thursday 5/11/06 Lesson Plan 27.6, 27.10-27.11 (The Wave Nature of Matter) The deBroglie Wavelength: Recall that the momentum of a particle with relativistic mass m’ is p’ = m’v = mv/(1-v2/c2)0.5, where “v” is, as before, the motion of inertial frame S’ in the x direction as observed in the S inertial frame and that, for a photon, p’ =h /c = h/. In general then, any particle may behave as a wave by p’ = h/= m’v = h/ (m’v). Assessment: Problems 36-38, p.855 The De Broglie Wavelength Applied to H-Atoms: If an electron circularly orbits the nucleus at a distance rn, and the electron has wave behavior, then 2rn = nnh/ (m’v), n= 1,2,3…. . The Bohr Radius: The circularly orbiting electron stays in orbit because the Coulombic m’vrn = nh/2 attraction between the nucleus (with Z protons) balances the centripetal force: F = k(Ze)e/rn 2 = m’v2/rn = m’(nh/(2 m’rn)2/rn So: rn = n2h2/(42m’kZe2 9 2 2 Where:k = 8.988 x 10 N-m /C -19 e = 1.6 x 10 C m’ = 9.11 x 10-31 kg h = 6.626 x 10-34 J-s So: rn = (0.529 x 10-10 )( n2/Z), m The potential energy of the electron in orbit is -F rn = -k(Ze)e/rn and the kinetic energy is 1/2m’v2 The total energy of the orbital is then: . En = 1/2m’v2 - k(Ze)e/rn En = 1/2m’(nh/(2 m’( (0.529 x 10-10 )( n2/Z) ) )2-k(Ze)e/((0.529 x 10-10 )( n2/Z) En = 1/2m’(nh/(2 m’( (0.529 x 10-10 )( n2/Z) ) )2-k(Ze)e/((0.529 x 10-10 )( n2/Z) En = - (2.17 x 10-18)Z2/n2, J En = - (13.6)Z2/n2, eV Assessment: Problems 48-51, p.856 Atomic Spectra: The Balmer series in the hydrogen spectra follow the equation: 1/ =(1.097 x 107)(1/22-1/n2), m-1 Electrons that fall back from an excited orbital n to the second orbital (n =2) release energy in the amount En –E2 = hc/ = - (2.17 x 10-18)(1/n2-1/22) 1/ =(2.17 x 10-18)(1/22-1/n2)/(( 6.626 x 10-34 J-s)*(3.00x 108 m/s)) =(1.097 x 107)(1/22-1/n2), m-1 Therefore, the de Broglie wavelength applied to the atom predicts the hydrogen spectra. Friday 5/12/06 The Electron—how was it 'discovered'? The what and the why of the Quanta. What is the Planck Quantum Hypothesis and why was it necessary? How does Einsein's explanation of the Photoelectric Effect extend the idea of the quanta and how does the Compton Effect confirm it? What was deBrogie's crazy idea and what is The Wave Nature of Matter? How do peculiarities of Atomic Spectra lead us to insights on The Structure of Atoms? ...and how does the Bohr Atom explain the Hydrogen Spectra? In what way does Bohr's theory of the hydrogen atom confirm the deBroglie Hypothesis? Lesson Plans: PHYSICS Monday Lesson Plan 28.1-28.2 (The “Matter Wave”) 5/15/06 Wavelength and Amplitude of the “Matter Wave”: Recall that the momentum of a particle with relativistic mass m’ is p’ = m’v = mv/(1-v2/c2)0.5, where “v” is, as before, the motion of inertial frame S’ in the x direction as observed in the S inertial frame and that, for a photon, p’ =h /c = h/. In general then, any particle may behave as a wave by p’ = h/= m’v = h/ (m’v) (The de Broglie wavelength). This is the wavelength of the “Matter Wave”. In order to determine the amplitude of the “Matter Wave”, the “wave function” or amplitude is used. Each particle is represented by a wave function position, time) such that * = the probability of finding the particle at that position at that time. Assessment: Problems 1and 2 and Question 1, p. 884 Wave function Properties: contains all the measurable information about the particle. * summed over all space = 1 (if a particle exists, the probability of finding it somewhere must be 1). is continuous. allows energy calculations via the Schrodinger equation. establishes the probability distribution in three dimensions. permits the calculation of the most probable or “expectation” value of a given variable. Finally, for a free particle is a sine wave, implying a precisely determined momentum and totally uncertain position. This last result is called the “uncertainty principle”. The Schrodinger Equation: The wave function is used in the Schrodinger equation. The Schrodinger equation plays the role of Newton’s laws and conservation of energy in classical mechanics. That is, it predicts the future behavior of a dynamic system. It predicts analytically and precisely the probability of events or outcome. The detailed outcome depends on chance, but given a large number of events, the Schrodinger equation will predict the distribution of the results. The time dependent Schrodinger equation for one spatial dimension can be viewed at http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/scheq.html Tuesday 5/16/06 Assessment: Discuss Fig. 28-4. Recall constructive and destructive interference with respect to the double slit “apparatus”. Derive that constructive interference occurs when dsin = mwhere m = 0,1,2 and destructive interference occurs when dsin = (m+1/2)Use the “de Broglie wavelength” in this equation. Lesson Plan 28.3-28.6 (The Uncertainty Principle and Quantum Numbers) The Heisenberg Uncertainty Principle: We cannot measure both the position and the momentum of an object precisely at the same time because the uncertainty in measuring position (x) is limited by the wavelength of light () used to make the measurement. The uncertainty in measuring the momentum (p ~ h/) is due to the fact that the interaction between the photon and the object results in either some or all of the photon’s momentum being transferred to the particle. The Heisenberg Uncertainty Principle is (x) (p) = h/(2) = 1.06 x 10-34 J·s = (E)(t) Wednesday 5/17/06 Assessment: Derive (x) (p) = (E)(t) from F = (p)/ (t) and (E) = F(x). Also, do problems 3-7, p. 885. The Principal Quantum Number n: Recall that the total energy of an electron in a Bohr orbital characterized by the integer n around a nucleus with Z protons was: En = - (13.6)Z2/n2, eV. The principal quantum number is n. (n is an integer between 1 and ∞). The energy of the electron in hydrogen is wholly characterized in this fashion. When there are more than one electron in the atom, the total energy is not characterized by this equation. The Orbital Quantum Number l: Recall that if an electron circularly orbits the hydrogen nucleus at a distance rn, and the electron has wave behavior, then 2rn = nnh/ (m’v), n= 1,2,3…. . m’vrn = |L| = n(h/2 where |L| = the magnitude of the angular momentum vector for the electron. When there are two or more electrons in the atom, the energy depends not only on n, but on l as well because the electrons not only interact with the nucleus, but they interact with each other as well. The orbital quantum number l is related to the magnitude of the angular momentum vector |L| as follows: |L| = (l(l+1))1/2 (h/2 The “quantum rule” to determine l is: l is an integer between 0 and (n-1) Assessment: Problems 13-17, p. 885 The Magnetic Quantum Number ml: The angular momentum vector L is quantized in both magnitude and direction when an external magnetic field is applied. If Lz is the magnitude of the projection of the vector L onto the “z” axis, then the quantized values for Lz are: Lz = ml(h/(2p)) where ml is the magnetic quantum number. The quantum rule to determine ml is: ml is an integer value between –l and +l. Assessment: Problems 18-22, p. 885. The Spin Quantum Number ms: Electrons are paired in “suborbitals” due to some property of electrons requiring pairing. The “fine structure” of each spectral line is evidence for this pairing. The quantum rule to determine the so-called spin quantum number ms is: ms is either +1/2 or -1/2. Assessment: Problems 23-26, p. 885 Lesson 28.7-28.9 (The Exclusion Principle, Hund’s Rule, Atomic Numbers) The Pauli Exclusion Principle: Each electron in an atom is characterized (or “named”) by a unique set of the four quantum numbers: n. l, ml, and ms . No two electrons in an atom can occupy the same quantum state. The principle also applies to protons and neutrons in “subatomic” physics. It does not apply to photons or -mesons. Hund’s Rule: Electrons are paired in “suborbitals” due to some property of electrons requiring pairing. The “fine structure” of each spectral line is evidence for this pairing. Electrons that have the same spin quantum number fill all the suborbitals of a “shell” first. Electrons with the opposite spin quantum number then fill the remaining positions in the suborbitals. The ground-state “shells” for the known elements correspond to the orbital quantum number and are named s, p, d and f. Assessment: Discuss the order in which electrons are filled in the periodic table of the elements. Also, do problem 31, p. 885. Emission Spectra and Atomic Number: Since the total energy of an electron with principle quantum number n around a nucleus with Z protons is (to a first approximation): En = (13.6)Z2/n2, eV, then a transition from an orbital for which n=2 to the innermost orbital for which n=1 will result in the release of a photon with energy E = - (13.6)Z2(1/22 -1/12) = hc/. It was determined by H.G.J.Mosely who died at the battle of Galipoli during WWI that the innermost (shell 1s) electrons effectively shield the outermost electrons from the nucleus, so Thursday 5/18/06 that the formula was empirically modified to E = - (13.6)(Z-1)2(1/22 -1/12) = hc/eV. Assessment: Problems 32-34 p.885. Also, determine the slope of a “Mosely Plot” ( Z vs. -1/2). Lesson Plan 28.10-28.12 (Fluorescence and Phosphorescence, Lasers, Holograms) Fluorecence and Phosphorescence: When ultraviolet photons strike electrons in fluorescent materials, the photon’s energy is absorbed by the electrons and the electrons are excited to higher energy states. Other, secondary photons are then emitted due to the transition(s) of the excited electrons to lower energy states. These secondary photons are often in the visible region. Excited states are usually unstable, so the electrons usually fall back to more stable (lower energy) states almost instantaneously. In some materials, however, the excited state is metastable. The excited electrons therefore take considerably longer to fall back to lower energy states. These materials are called phosphorescent. Assessment: Observe fluorescence using a “black light” and materials such as white paper, vitamin B-12 dissolved in vinegar, ground spinach in ethanol for chlorophyll, detergent, postage stamps, minerals (fluorite, calcite, gypsum, opal, agate, ruby, talc, quartz and amber), body fluids (blood, urine and semen), club soda or tonic water. Observe luminescent (i.e., phosphorescent) paints and zinc sulfide. Compare and contrast fluorescence and phosphorescence. Lasers: Light amplification by stimulated emission of radiation is achieved first by exciting most of the electrons in the material so that emission of photons will dominate over absorption of photons and secondly by insuring that the excited state is a metastable state so that the excited electrons only transition to lower energy states when they are stimulated by other photons to do so. Stimulated emission produces photons that are of the same frequency; are exactly in phase, and; that move in the same direction. Lasers are used in bar-code readers and in CD players. NEVER LOOK DIRECTLY INTO THE BEAM OF A LASER. An unspread beam from a small 0.5 milliwatt laser is well above the ANSI standard for eye safety. Assessment: How do CD players work? Go online to http://www.howstuffworks.com/cd.htm. Explain why a thin aluminum film is applied to the polycarbonate plastic disc. What is the width of the spiral track of data? The pits on the aluminum side look like bumps on the polycarbonate side (which is the side that the laser reads from). What do the bumps do? What are the components of a CD player? What is the fundamental job of the CD player? Why does the tracking system slow the speed of the laser pickup as the laser moves outward on the spiral track? Why does a scratch or a speck on a CD cause a major error on a CD? How does the drive recover from this? Holograms: Interference of laser light (that originates from a common source) in photographic film produces holograms. The interference patterns record the intensity and relative phase of the light that was reflected from the object exposed to the laser light. Assessment: Observe white light holograms. Notice the ability to view around the object as the film is turned. How does a holograph differ from a photograph? Inspect the holograph closely with a magnifying lens. What do you see? How are holographs used? Friday 5/19/06 Lesson Plans: PHYSICS Monday Lesson Plan 29.1-29.3 (Molecules and Solids: Covalent and Ionic Bonds; Binding Energy; Activation 5/22/06 Energy; Weak Bonds) Covalent Bonds: The diatomic hydrogen molecule (H2) is formed from two hydrogen atoms that bond together (H-H). The quantum numbers (n, l, ml and, ms) for the electron of the first hydrogen atom are 1,0,0,+1/2 and the quantum numbers for the second hydrogen atom are 1,0,0,-1/2. Since the spin quantum numbers are different, the two hydrogen atoms are now in different energy states. That is, the 1s energy level has split into two energy levels as the atoms approach each other. The electrons can now move about in the space of either atom. This increase in the space available for either electron (x) will, according to the uncertainty principle ((x) (p) = h/(2) = 1.06 x 10-34 J·s = (E)(t)), reduce the uncertainty in the momentum (p) . Since E = (p) (x) / (t), then reducing p will reduce E in an inertial frame (absence of Tuesday acceleration). In short, bonding lowers the energy of the system of two atoms. Both electrons are attracted to both nuclei equally. This type of bond is called a non-polar covalent bond because of the symmetry of the distribution of the probabilistic matter waves (for electrons) that eliminates separation of spatial charges. When the bonding atoms are different, however, the symmetry may be uneven. This type of bond is called polar covalent. Electronegativities arte assigned to each element. When the difference in electronegativities of the elements involved in a bond is less than 1.5, then the bond is covalent. If that difference is less than 0.3 for a covalent bond, then the bond is non-polar covalent, otherwise it is polar covalent. If the difference on electronegativities is greater than 1.5, he bond is ionic. http://en.wikepedia.org/wiki/Electronegativity Ionic Bonds: The negatively charged electrons are attracted to the positively charged nuclei. When there is a spherically symmetric “closed” shell, such as a filled “s” shell, the nucleus is partially shielded by electrons. That is, any electrons located in higher energy levels will only “feel” part of the positive charge from the nucleus. When the outer shell is unsymmetrical (e.g., a “p” shell is unsymmetrical) then the unshielded partial charge of the nucleus may be fully “felt” by a single electron at times. This unequal attraction to the nucleus between highly electronegative atoms and weakly electronegative atoms leads to ionic bonding. Binding Energy and Electrostatic Forces: Recall Coulomb’s law that describes the electrostatic force due to two point charges is F=k(Q1Q2/d2) , where k = 8.988 x 109 Nm2/C2, and that the potential energy is PE = F·d = k(Q1Q2/d). The separation distance “d” is the distance between the charges. When Q1 is negative and Q2 is positive, PE is negative and the electrostatic force is attractive. Note that electrons and nuclei repel each other, but nuclei and electrons attract each other. The interatomic distance “r” is not the same as the distance between charges “d”. The potential energy is calculated from the sum of the electronelectron repulsion force, the nucleus-nucleus repulsion force and the electron-nuclei attraction force. When the potential energy is most negative (or least positive), the molecule is at its lowest energy state. The value of the potential energy at this value of the interatomic distance, r0, is the binding energy of the molecule. Binding energies within a molecule formed from two atoms or ions are typically on the order of 2 to 5 eV. Assessment: Problems 1- 4, p. 912 Activation Energy: At distances greater than r0, the (positive) electron-electron repulsion force may dominate. If the potential energy forms a local maximum, then this maximum is called the activation energy. Activation energy arises because of the spatial configuration of electrons of the bonding atoms. It can be manipulated by rearranging the configuration of the electrons. Catalysts and enzymes rearrange the configuration of electrons thus allowing the activation energy to be reduced. Assessment: Demonstrate sparks that cause the Bunsen burner to initiate combustion. Weak (van der Waals) Bonds: A polar molecule is one that has a spatial separation of electrical charges. Polar molecules therefore have permanent (or induced dipoles) that can attract each other. The potential energy for these dipoles between polar molecules may be described as PE = k(Q1Q2/d6) resulting in weak bonds typically on the order of 0.04 to 0.3 eV. http://www.chemguide.co.uk/atoms/bonding/vdw.html Assessment: Likes dissolve Likes means polar solvents dissolve polar solutes and vice-versa. Observe this with various solvents and solutes. Why does glucose dissolve in water but cyclohexane does not? Lesson Plan 29.4-29.6 (Molecular Spectra, Molecular Rotation; Molecular Vibration; Bonding in Solids; Band Theory of Solids) 5/23/06 Molecular Spectra: When photons are absorbed by electrons, the electrons can undergo transitions between electron energy levels. In molecules, the electrons reside in orbital, rotational and vibrational energy levels and, when struck by photons, can move to higher orbital, rotational and vibrational levels. After the collisions, the excited electrons fall back to the lowest possible orbital, rotational and vibrational levels emitting other photons of energy equal to the differences in energy between the higher and lower orbital, rotational and vibrational levels. Molecular Vibration: The strong bond between two atoms or ions in a molecule is like a spring with spring constant k. Recall that the potential energy of a spring is PE = =(1/2)·kx2 where |x| = |r-r0|. A perturbance of the bond length by an amount |x| will cause the potential energy of the bond to be perturbed by this amount. A photon of energy h will be emitted due to vibration of the bond only when the perturbance of the potential energy of the bond is equal to this amount. Therefore the separation in energy between different, adjacent vibrational levels = Evib = h. Each vibrational energy level is characterized by Evib = (+1/2)h, where is the vibrational quantum number ( is an integer greater than or equal to 0). However, the selection rule for any transition between vibrational energy levels is that = ± 1. Molecular Rotation: Recall that for a system of particles the translational KE = (mv2/2), and ar = v2/r=(r)2/r=r, so rotational KE = Erot = ( (mr2))·2/2=I2/2, where I = (mr2) is the moment of inertia of the system of particles. Since the magnitude of the angular momentum vector for the system of particles is defined as |L| =I then Erot = |L| 2/(2I). The rotational angular momentum quantum number l is related to the magnitude of the angular momentum vector |L| as follows: |L| = (l(l +1))1/2 (h/2 1/2 Hence, Erot = [(l(l +1)) (h/22/(2I) where, l is an integer greater than 0. The transition energy between two rotational energy levels, say l and l - 1, is Erot = [(h/22/(2I)·[ l(l +1) - l(l -1) ] = [(h/22/(I)·[ l ]. However, the selection rule for any transition between rotational energy levels is that l = ± 1. Also, molecules have band spectra whereas isolated atoms have line spectra. The upshot is that, for every orbital energy level (say 2s or 3p, etc.) in isolated atoms, for molecules, liquids and, solids there will be several vibrational and rotational levels corresponding to the vibrational and rotational angular momentum quantum numbers and l. http://hyperphysics.phy-astr.gsu.edu/hbase/molecule/vibrot.html#c1 Assessment: Problems 8-10 and 15, p. 913 Bonding in Solids: Ionic, covalent and metallic bonds are the strong bonds between atoms in solids. Band Theory in Solids: When a large number of atoms come together to form a solid, then each of the original energy levels splits into as many energy levels as there are atoms. The original atomic levels become a band of atomic levels. The highest energy band containing electrons for a good conductor is only partly filled. The highest energy band is called the valence band. The valence band for an insulator is completely filled. Above the valence band and separated by an energy gap is the conductance band. The conductance band has no electrons in it usually. Any electrons that enter it will conduct electricity because the band would only be partly filled. The energy gap between the valence band and the conductance band is usually high (~5 eV) for insulators and low (~ 1 eV) for semiconductors. Once electrons acquire sufficient energy to cross the energy gap (by absorption of a photon or by an external electric field or by thermal heating), the valence band becomes partly filled thus allowing it to conduct electricity as well. A solid is transparent to light of frequency when the energy gap > h. This is because the light has insufficient energy to remove an electron from the valence band into the conductance band, so the photon is not absorbed. Conversely, if the energy gap < h, the photon that collides with an electron is absorbed and the material is opaque to the light. http://hyperphysics.phy- astr.gsu.edu/hbase/solids/band.html Assessment: Problems 19-23, p. 913. Relate band theory to X-Ray Imaging. Wednesday 5/24/06 Lesson 29.7 (Semiconductors and Doping) Doped Semiconductors: . http://hyperphysics.phy-astr.gsu.edu/hbase/solids/dope.html#c5 In n-type material (As in Si), there are electron energy levels near the top of the energy gap thereby narrowing the electron gap so that electrons can be easily excited into the conductance band. In p type materials (Ga in Si), extra holes near the bottom of the energy gap allow excitation of valence band electrons into the holes thereby leaving the valence band partly filled so that the valence band can conduct electricity. http://hyperphysics.phy-astr.gsu.edu/hbase/solids/dsem.html Assessment: Problem 26, p.913. Thursday 5/25/06 Lesson Plan 29.8-29.9 (Semiconductor Diodes, Transistors and Integrated Circuits) What is a Diode?: Thermionic or gaseous state diodes are a vacuum tube device. The incandescent light bulb is a vacuum tube. In the light bulb a hot filament releases electrons into the vacuum (thermionic emission). This negatively-charged cloud is called a space charge. If a positively charged plate is included inside the vacuum tube, the space charge will be attracted to it. Thus a current of electrons flow from the filament to the plate. Current cannot flow in the other direction because the plate is not heated and cannot emit electrons. A diode is a device that conducts electricity in only one direction. http://en.wikepedia.org/wiki/Vacuum_tube Assessment: Review the development of the diode. Include rectification of a-c current; the triode and grid bias; “A”, “B” and “C” batteries to control bias; the tetrode and the screen grid; the pentode and the suppressor grid; hexodes, heptodes, octodes and nonodes in superhetorodyne receivers; combination tubes, and; reliability issues of vacuum tubes. Demonstrate the Thompson tube. Discuss the cavity magnetron. http://en.wikepedia.org/wiki/Magnetron The p-n Junction Diode: http://en.wikepedia.org/wiki/Image:Diode_symbol.svg When an n-type semiconductor is joined to a p-type semiconductor, electrons diffuse from the ntype to the p-type leaving the n-type positively charged relative to the p-type. A forward bias across the junction (positive battery terminal to p-type semiconductor) allows electrons to flow from p-type to n-type. A reverse bias (negative battery terminal to p-type semiconductor) will stop the electron current. A large reverse bias will cause the atoms to ionize at the junction however. The Zener diode is designed to operate at the breakdown voltage. Assessment: Review diodes, Zener diodes, Tunnel diodes, LED’s, photodiodes, SCR’s, Varicaps and the Schottky diode. Problems 27-30, p. 913. Transistors and Integrated Circuits: http://en.wikepedia.org/wiki/Bipolar_junction_transistor Friday 5/26/06 The pnp and npn junction transistor are bipolar because they use both electrons and holes to carry the main electric current. The emitter-base junction is forward biased and the base-collector junction is reverse biased. A transistor is a solid state triode. Assessment: Review a water analogy http://www.satcure-focus.com/tutor/page4.htm Problem 35, p. 914. Lesson Plans: Physics Monday 5/29/06 Tuesday 5/30/06 Holiday Lesson 30.1-30.3 (Nuclear Physics: The Nucleus and Nuclear Reactions) Wednesday 5/31/06 Handouts: Lesson worksheet 22.1 Transparencies: T 130 and T 131 Nucleons are protons and neutrons. The nucleus of the atom is made of protons and neutrons. The atom is called a nuclide in nuclear chemistry. The atom is made of protons, neutrons, and electrons. Assessment: Lesson worksheet problem 1 Mass Defect and Binding Energy per Nucleon: the difference between the mass of an atom and the masses of its protons, electrons and neutrons. The mass defect is equal to the nuclear binding energy through the equation “E=mc2”, where “c” is the speed of light. Assessment: Lesson worksheet problems 2 and 3 Nuclear Stability: Nuclei for which the neutron to proton ratio is between 1.1 and 1.5 are stable. Protons in the nucleus electrostatically repel one another so much so that the nucleus becomes unstable when there are large numbers of protons in it. Assessment: Lesson worksheet problems 4 and 5 Nuclear Reactions: a reaction that affects the nucleus of the atom. When the reaction results in a change of the number of protons of a nucleus, the reaction is a transmutation reaction. The total of the atomic numbers and the mass numbers must be equal on both sides of the equation. Assessment: Lesson worksheet problems 6 and 7 Lesson 30.4-30.10 (Radioactive Decay) Handouts: Lesson worksheet 22.2, TM125A and TM 126A Transparencies: T 132 and T 133 Types of Radioactive Decay: Radioactive decay is the spontaneous disintegration of the nucleus into a slightly lighter nucleus, accompanied by emission of particles or electromagnetic radiation. Unstable nuclei undergo radioactive decay. Assessment: Lesson worksheet problems 1 and 2 Half Life (t ½): This is the time required for ½ of the atoms of a radioactive nuclide to decay. The decrease in the number N of radioactive nuclei per unit of time t is proportional to the remaining number of nuclei: lim t∞N/t) = dN/dt = -N where is the “decay constant . This is called the “radioactive decay rate”. Rearranging and integrating this equation gives: N = N0e-t Where N0 is the number of radioactive nuclei initially at t= 0. When N = 1/2N0, then t= t1/2 Assessment: Lesson worksheet problems 3 and 4 Decay Series: This is a series of radioactive nuclides produced by successive radioactive decay until a stable nuclide is reached. The heaviest nuclide of each series is called the “parent nuclide” while those nuclides produced by radioactive decay are called the “daughter nuclides”. Assessment: Lesson worksheet problem 5 Lesson 30.11-30.13 (Radioactive Dating, Tunneling and Detection of Radiation) Radioactive Dating: Nitrogen in the atmosphere undergoes the nuclear reaction: 14 N + 1 n 14 C + 1 H 7 0 6 1 4 Thursday 6/1/06 Carbon-14 is radioactive ( t1/2 = 5730 yr.) and decays by the reaction: 14 C 12 C + 2 ( 1 n) 6 6 0 Friday 6/2/06 The ratio of carbon-14 to carbon-12 in the atmosphere and all living organisms is 1.3 x 10-12. When an organism dies, respiration ceases so the ratio of carbon-14 to carbon-12 decreases due to radioactive decay. Assessment: Problems 35-40, p. 941 Tunneling: Radioactive decay only occurs in reactions that have a mass defect. The rate of decay depends on the nuclear attraction for the decay particle within the nucleus and the electrostatic repulsion between the decay particle once freed from the nucleus. Some of the decay particles will always have sufficient energy to overcome both of these forces. Detection of Radiation: Determine how a Geiger Counter Works. Also review cloud chambers and bubble chambers. Lesson Plans: Physics Monday 6/5/06 Tuesday 6/6/06 Wednesday 6/7/06 Thursday 6/8/06 Friday 6/9/06 Lesson 31 (Nuclear Fission and Nuclear Fusion) Handout Chapter 31 Worksheet Fill in all blanks on the worksheet RETURN YOUR PHYSICS TEXT BOOKS BETWEEN 6/6/06 AND 6/8/06. Review of Nuclear Physics for the Physics Final Chapter 30—Go over worksheets 22.1 and 22.2 Last Call for Delinquent Work—All exams that were given this semester must be submitted for grading by today. No exams will be accepted after today PHYSICS FINAL EXAMINATION (REQUIRED TO PASS THE SEMESTER) A passing semester grade requires that you take the final examination. Period 5 Student Tatiana Addison Marianna Baliesteros Samantha Barbosa Brenda Botello Isaac J. Brown Jasmine M. Brown Leticia Casteneda Cinthya C. Cuevas Feliciano Echeverria Breanna M. Hughes Danny Johnson Dawnetta Jones Kevin T. Jones Abbas Khalid Agueda V. Mallari Sonia Martinez Stephanie A. Mata Ramon Miranda Seat No. Contact No. Address Parent 1 15SEP09: Lab: Measurement A 2 C 29 C 5 C 8 9 C 10 11 13 18 A Tonya Webb (Mother (510)393-0779 cell, work (510) 3930779); father (work, (510) 6828202) 4517 Adeline St, Emeryville 94608 TONYA WEBB 1054 MARKET (925)681-1701 JANICE WEBB 655-2084 166426 15 20 A 16 A 23 24 25 C Isamar Montoya Rafael Navarro Rickeya Ooten Duana D. Palmer Juan Perez 26 Oscar Rios 3 17 A 22 A 21 6 Robby Ross Damiah D. Smith Andrea Vargas Liliana P. Vargas Lauren A. West 31 A 30 A 7 C 19 Period 6 Student Contact No. Trey A. Brown 7 Maria E. Casillas 8 Priscilla P. Enninful 12 Hector Estrada17 Torres Jennifer Fabian 1 Arlene Gonzalez Aquetea L. Goodman Carleena Henderson Address Parent 16SEP09: Lab: Measurement A A 14 3 A 16 Auric Horneman Alejandra J. Moran Chrissean S. Moss Kassandra Perez Janiah J. Smith Leilani Victoria Tucker Orlando Urias Reginald Whitaker Angenique N. Seat No. A 19 11 A 27 32 4 A A A 2 36 15 A Williams