BioPlex protocol
advertisement

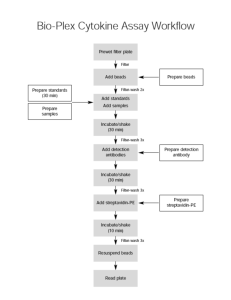
Dart Lab’s Bio-Rad Luminex Record Sheet For Bio-Rad’s protocol, go to www.Bio-Rad.com. Analytes: Human 6-plex IL-2, IL-5, IL-9, IL-10, IL-13, VEGFa Supplier: Bio-Rad I mixed 6 vials of beads & det ab PI: Magnetic kit 1 Plate: 96 wells (63 sample wells) Plate: Pl 1 of 1 Type of Samples: Date: # sample wells on this plate: 63 Total # wells on this plate: 96. Standards=24 Samples=63; Blanks=3; Controls=6 Standards Diluent: Samples this plate: Pl 1: 96 (24 standards; 63 samples; 3 blanks, 2 sets of control in triplicates (1/3, 1/400 in Assay buffer) Kit Used: Medium: Calibration: BROAD kms Preparation of 10X Beads: I mixed 6 vials of beads Diluent: Assay Buffer A Volume to be added to wells: __50___ l # wells 96 10x Beads (36ul) 575x6=3450 ul Assay buffer (24ul) 2.3 ml Bead count: Bead Count per 400 small squares: Side 1: ______ Side 2: ______ Total Volume (60ul) 5.75 ml Mean: ______ Use the following formula to calculate if there are sufficient beads for the assay: Mean of duplicate bead count x 1000 (mm3 to cc3 conversion factor) x volume beads added per well (ul) 1 sq. mm (400 small squares) x 0.1 mm (depth) x Number of Cytokines x 1000 i.e. Mean of duplicate bead count x volume beads added per well (ul) 0.1 x number of cytokines = beads/cytokine/well Example: if doing a 4-plex, have counted 56 and 52 beads in 400 small squares, and 1 Dart Lab PI Date of Expt Jacqueline Smith Nov. 11, 10 Kathy Smith Dart Lab’s Bio-Rad Luminex Record Sheet added 50 ul beads per well: Mean = 54 Calculation: 54 x 50 = 6750 beads/cytokine/well 0.1 x 4 ______ x _50___ = beads/cytokine/well 0.1 x 6 QA/QC: Beads/cytokine/well must be greater than 2000 to continue. Preparation of Standard Curve Made 1 set of standards. Diluent used: ________ This is the most important part of the procedure. Be really accurate with volumes, i.e., no bubbles in tip. 1. Lyophilised standard 1 Gp I reconstituted with _500_ ul _(diluent)_______, resulting in 22147 pg/ml IL-2. Vortex gently 1-3 sec and incubate on ice for 30 min. Equilibrate 20 min. at RT. Vortex gently. This master mix will serve as Standard 1 in the Broad PMT setting standard curve. Standard 1 = _256_ l of stock + ____ __144____ of diluent. Time: ___________ Equilibrate:_______ Standard 2 = _100_ l of standard 1 + _____300____ of diluent. Start dilutions: ____ Standard 3 = _100_ l of standard 2 + _____300____ of diluent. Standard 4 = 100_ l of standard 3 + _____ 300____ of diluent. Standard 5 = _100_ l of standard 4 + _____300____ of diluent. Standard 6 = _100_ l of standard 5 + _____300____ of diluent. Standard 7 = _100_ l of standard 6 + _____300____ of diluent. Standard 8 = _100_ l of standard 7 + _____300____ of diluent. Vol of Standard to add to wells: __50ul__ I ran 1 set of Blanks: 3 wells @ 50 l/well of_____. Preparation of Controls (Spikes) Add 50 ul to each well. Diluent: Assay Buffer. QA/QC: A high and low spike should be also be added to each plate as an internal Quality Control. As of 20 Apr 06 Dart Lab has used pooled supernants from Human DC experiments as Biological Controls. 1 Dart Lab PI Date of Expt Jacqueline Smith Nov. 11, 10 Kathy Smith Dart Lab’s Bio-Rad Luminex Record Sheet Make enough Control for 1 plate: To make a 1/3 and 1/400 dilution and add 50ul to 6 wells: Diluent: Assay Buffer. 1/3: Of the approved Hu DC/PBMC exp’t aliquot, take 100 ul + 200 ul Assay Buffer = 300ul 1/3 spike. Plate 50ul in triplicate. Use 10ul for 1/400 spike. 1/400: 1/3 x 1/133: Take 10 ul 1/3 spike + 1.32 ul Assay Buffer = 1.33 ul 1/400 spike. Plate 50 ul in triplicate. Dilutions: High 1/3 __X__ Low 1/400 ___X___ . Vol of Spike to add to wells: _____50____ l. Preparation of Samples Vol of Samples to add to wells: _50__ l. Are any samples diluted? _No.__ Indicate on BioPlex software protocol. Incubation time of beads and samples (30 min): Pl 1 From ______________ to __________________ Preparation of Biotinylated Detection Antibody (DA): Diluent: Detection Antibody Diluent A Amount to add: 25 ul/well # wells 96 10x Det Ab (18.75ul) 300x6=1800ul Det Ab Diluent (12.5ul) 1.2 ml Total Vol (31.25ul) 3.0 ml Incubation time of detection antibody (30 min): Pl 1 From ______________ to _______________ Preparation of Streptavidin-PE Working Solution: Diluent: Assay Buffer Amount to add: 50 ul/well # wells 96 100x Strep-PE (0.625ul) 60ul Assay Buffer (62ul) 5.94 ml Total Vol (62.5ul) 6.0 ml Incubation time of Streptavidin-PE (10 min): 1 Dart Lab PI Date of Expt Jacqueline Smith Nov. 11, 10 Kathy Smith Dart Lab’s Bio-Rad Luminex Record Sheet Pl 1 From ______________ to __________________ Calibration of Bio-Plex Reader: Broad ___X____ Time Plate 1 Read Start: _________ Finish: ________ Remove stickers from vials and tape here: catalog number lot number expiration dates Beads Standards Detection antibody Streptavidin-PE Spike 1 Dart Lab PI Date of Expt Jacqueline Smith Nov. 11, 10 Kathy Smith