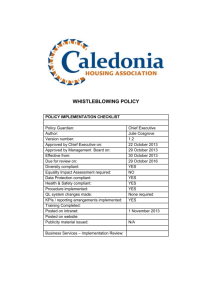
Supplementary material
advertisement

SUPPORTING INFORMATION Biogeographical affinities of the New Caledonian biota: a puzzle with 24 pieces Michael Heads Journal of Biogeography Appendix S1: Supplementary notes on New Caledonian biogeography This appendix includes notes on methods and on each of the 24 tracks described in the main text, an analysis of the terrestrial isopods of New Caledonia, and documentation of correlated local and global patterns for New Caledonian taxa. Methods in historical biogeography Metapopulations and dispersal Van Balgooy et al. (1996, p. 210) concluded their discussion of Pacific Cupanieae (Sapind.) by writing that: ‘One can only detect that dispersal is involved, because the geological history of the Pacific is not consistent with the idea that all Pacific island groups together with Australia have formed one united area which has broken up in the course of time’. This denies ordinary, local dispersal in a metapopulation, but accepts extraordinary long distance dispersal. Modern centre of origin/dispersal theory In their study of Pachycephalidae, Jønsson et al. (2010) calibrated the clock with endemics on Sangihe I., formed at 2-3 Ma, and Tanimbar I., formed at 1 Ma. To corroborate this first calibration, the authors used another rate, but this was also based on the assumption that an island endemic (Mimus graysoni) could be no older than its island (Socorro I. off western Mexico; Arbogast et al., 2006). As with the Indonesian islands, Socorro I. also occurs near an active plate margin and, again, the assumption that its endemics are no older than the island is very likely to give dates that are much too young. Any ‘corroboration’ between the two calibrations is meaningless. Although the spatial and chronological analyses of Jønsson et al. (2010) are not accepted here, the molecular phylogeny itself is of great interest. The main clade in Pachycephala comprises three branches in an unresolved trichotomy: P. caledonica of New Caledonia (Grand Terre only, not Loyalty Is.), P. soror of New Guinea, and a 1 large group ranging from Bangla Desh to Samoa, including New Guinea and New Caledonia (Grande Terre and Loyalty Is.). For P. caledonica, Jønsson et al. (2010) proposed a centre of origin in New Guinea and colonizing flights to New Caledonia, Bangla Desh, Samoa etc. But the centre of origin can be interpreted instead as a centre of differentiation in a widespread Bangla Desh – Samoa ancestor and long distance colonizing flights are unnecessary. Following initial vicariance of the three clades there has been secondary, local overlap in New Guinea and Grande Terre caused by terrane accretion, population expansion, or both. In Pachycephala the second New Caledonian species is a form in the large Bangla Desh – Samoa group cited above, a subspecies of P. rufiventris found on Grande Terre. This was not sampled in the molecular study but probably has its closest relatives in Australia. Any overlap with P. caledonica within Grande Terre (distribution maps do not seem to be available) could be due to range expansion within the island following initial vicariance between P. caledonica and the large group that includes P. rufiventris. The third New Caledonian Pachycephala also belongs to the large group cited above, and is a member of the diverse P. pectoralis species complex. This has a phylogeny: (Vanuatu (Bismarck Archipelago (Solomon Is. (Tanimbar I. (Australia))))). It also occurs in New Caledonia, but only on the Loyalty Is. and not on Grande Terre. Jønsson et al. (2010) interpreted the phylogeny as representing a colonization of the Melanesian arc from New Guinea (their Fig. 4 indicates colonization of Vanuatu from New Guinea via long distance dispersal north of the Bismarck Archipelago/Solomon islands, then the Bismarck Archipelago from New Guinea, then the Solomon Islands from New Guinea). Finally, there was a colonization of Australia from a centre of origin from somewhere in New Guinea/Pacific islands, where the basal grade occurs (cf. Rhipidura: track 6; monarch flycatchers, Filardi & Moyle, 2005). None of these dispersal events are necessary as the species are allopatric. In addition, dispersal does not explain the absence of the group from Grande Terre. The simplest explanation of the phylogeny is that it represents a series of differentiation events in an ancestor already widespread in Australia and the Pacific islands, including the Loyalty Is. but not Grande Terre. The biogeographical boundary between the Loyalty Is. and nearby Grande Terre is a standard one (cf. the plant Cyrtandra, Fig. 9) but is not explained in a dispersal model. Documentation for the New Caledonia tracks For each area, notes on the treatment in the main text are given followed by information on lower plants, seed plants, invertebrates and vertebrates. Family names for seed plant families are abbreviated. Data is included for liverworts (Miller et al., 1983), mosses (Miller et al., 1978), higher plants (Council of Heads of Australasian Herbaria, 2009), Diptera (Evenhuis, 2005a), spiders (Platnick, 2007), birds (Dickinson, 2003), and other groups as cited. Molecular evidence was used wherever available, but only for phylogenetic and geographical information; the chronologies of molecular clock studies were not accepted. Patterns are usually cited only if the groups they belong to seem to be reasonably well-collected. For example, disjunctions are usually only recorded if closely related taxa have been collected in the gap. 1. New Caledonia – south-west Indian Ocean Notes on the main text 2 Although Davis et al. (2002) invoked long-distance dispersal for Acridocarpus from Madagascar to New Caledonia, Davis et al. (2004) insisted that wind-dispersed Malpighiaceae fruit such as those of Acridocarpus ‘do not easily travel for long distances over water’ which argues against long-distance dispersal from Madagascar to New Caledonia, 10 000 km away. Another winddispersed member of Malpighiaceae, Hiptage myrtifolia, is a Fiji endemic most closely related to H. luzonica of the Philippines and Sulawesi, 6000 km away (Jacobs, 1955). Davis et al. (2002) considered the possibility that Acridocarpus has gone extinct in Australia due to aridity, but this is unlikely in a group that has flourished in Africa. Instead, populations may have been extirpated during subsidence of basins around the Timor Sea – Torres Strait region. Despite the problems with both long-distance dispersal and extinction, Davis et al. did not mention the main evidence for a vicariance explanation, the fact that the south-west Pacific Ocean – south-west Indian Ocean connection is a standard pattern. Good’s (1950) account of ‘Madagascar and New Caledonia’ is a useful discussion of the topic but dispersalists have seldom mentioned the pattern; Thorne (1965) wrote simply that ‘Little significance can thus be accorded those taxa restricted to New Caledonia and the Malagasian region beyond their relict status...’. This does not account for the frequent vicariance observed between New Caledonia – south-west Indian Ocean groups and their northern relatives. Lower plants In algae, Millar & Bolton (2004) cited several species restricted to eastern Australia (New South Wales) and South Africa. The liverwort Cololejeunea ceatocarpa is in Réunion, New Caledonia and Hawaii, and C. cuneata is in Mauritius and New Caledonia (Miller et al., 1983). In liverworts, Cololejeunea cardiocarpa is in New Caledonia, Madagascar, southern central and East Africa, and southern United States/Greater Antilles. Seed plants In Phyllanthus (Phyllanth.), matK sequences link some New Caledonian members of subgen. Gomphidium with another New Caledonian clade of Phyllanthus (groups 6 and 7 of Aubréville et al., 1967-) (Kathriarachchi et al., 2006). The ITS tree instead places the New Caledonian Gomphidium with the Madagascan P. betsileanus (bootstrap support 92%). Kathriarachchi et al. (2006) regarded the New Caledonia – New Caledonia tie as ‘biogeographically more plausible’ than the New Caledonia – Madagascar disjunction and so they removed the ITS data of the Madagascan species from the combined (ITS and matK) matrix, nevertheless, the ITS affinity again fits the standard pattern. Groups centred around the Indian Ocean include the trees in tribe Caletieae (Picrodendr.) of South Africa, Australia, Malesia, New Caledonia and Fiji (Radcliffe-Smith, 2001). The group is well-represented in New Caledonia, with four genera: Austrobuxus, Canaca, Longetia, Scagea. The last three are endemic to New Caledonia, while Austrobuxus has most of its species (14 out of 20) there. In other angiosperms, Arthropodium (Antheric.) is in Madagascar, Australia, New Zealand, New Caledonia, and New Guinea (van Balgooy, 1971). Pigea etc. (= Hybanthus p.p.) (Viol.; Fig. 13) has a related pattern. Disjunct clades between the south-west Pacific and south-west Indian Oceans include a group in Crossosomatales, Geissolomataceae (south-west South Africa) and its sister clade Strasburgeria (New Caledonia)/Ixerba (New Zealand). Korthalsella salicornioides (Visc.) is in Madagascar, New Zealand and New Caledonia (I. des Pins and Kouaoua, southern Grande Terre) (Aubréville et 3 al., 1967; Molvray, 1997). The mainly Malesian/Pacific species Geniostoma rupestre (Logan.) has an outlier in the south-west Indian Ocean, G. r. var. borbonicum of the Mascarenes. It is closest to G. r. var. glaberrimum from New Caledonia and distinguishing the two ‘is often extremely difficult’ (Conn, 1980). In other plants (data from Aubréville et al., 1967-), Agrostophyllum (Orchid.) is in Malaysia, Indonesia, Melanesia and Polynesia, with a single species in the Seychelles (A. occidentale). This last species is ‘very similar’ to the single species in New Caledonia. Cohnia (Lomandr.) is often accepted as a separate genus for the free-tepalled species otherwise placed in Cordyline. It occurs in New Caledonia and the Mascarenes (Conran, 1998). Cynorkis fastigiata (Orchid.) is known from the south-west Pacific (Fiji and Futuna) and the south-west Indian Ocean (Madagascar, Mascarenes, Seychelles) (Renz & Vodonaivalu, 1989). Cossignia (Sapind.) is in north-eastern Queensland, New Caledonia and Fiji, and disjunct in the Mascarenes (Smith, 1979-1996). Albizia guillainii of New Caledonia is not related to Asian or Australian congeners, but to African ones (sect. Zygia). Invertebrates The large landsnail Placostylus ranges widely around the Tasman and Fiji basins (Lord Howe I., northern New Zealand, New Caledonia, Vanuatu, Fiji, and the Solomon Is.). Its sister, Bothriembryon, is in Tasmania and south-western Australia. The sister group of the pair is Prestonella of southern Africa (Herbert & Mitchell, 2008). The authors supported a vicariance interpretation for the pattern. In the leafhopper family Myerslopiidae (Homoptera), one tribe (Sagmatiini) ranges in Madagascar, eastern Queensland, and New Caledonia, one is in vicariant in New Zealand/Chile, and one is endemic to Juan Fernandez Is. (related families are mainly diversified in continental Africa) (Hamilton, 1999). Hamilton concluded that the New Zealand – Chile tribe, at least, is ‘virtually certain’ to date from the break-up of Gondwanaland and so it would seem likely that the Sagamatiini are also a Mesozoic group. The spider genus Pachyballus (Salticidae) is restricted to New Caledonia and Africa (in the south, east and west). New Caledonian members of Philoliche (Diptera: Tabanidae) are ‘unequivocally’ related to Afrotropical species and have their closest relative in P. pennata of Mauritius (Burger, 1995). Aselgeoides (Homoptera: Fulgoroidea) occurs in New Caledonia, and also in the Seychelles, Madagascar and Africa (Fennah, 1969). In Psocoptera, species of Phlotodes ‘Group II’ occur on New Caledonia, Fiji and New Guinea, and also in Madagascar (Thornton, 1981). A trochiform landsnail of Norfolk Island, south of New Caledonia, is closest to a Madagascan species (F.M. Climo, pers. comm., 2002). The bivalve genus Funafutia (Lucinidae) occurs in the south-west Pacific (Queensland, New Caledonia, Kiribati, and Tuvalu) and also around Madagascar (Glover & Taylor, 2001) but is absent from western Australia (where other lucinid genera such as Pillucina occur). The centipede Dichelobius shows a pattern: (Queensland (Western Australia + New Caledonia)) (Edgecombe & Giribet, 2004) which may be related to this pattern, and the spiders Epimecinus (Desidae) and Syrorisa (Desidae), and the ostracod Vestalenula marmonieri (Martens & Rossetti, 2002) are also restricted to New Caledonia and Western Australia. A New Caledonia/India disjunction may also be related and is shown by several spiders: Penionomus (Salticidae) of New Caledonia and India, Stergusa (Salticidae) of New Caledonia and Sri Lanka, and Thomiscus leucaspis (Thomisidae) of New Caledonia and India, with the gap filled 4 by T. spectabilis: India to Australia. Thomiscus otherwise ranges east to Java/Sulawesi, and is also in the Americas. Vertebrates Fricke (2002) cited tripterygiid fish species with ‘bipolar west-east’ distributions, disjunct between the west Indian Ocean and the west Pacific Ocean, and ‘tripolar’ species (such as Enneapterygius elegans) which range around the Indian Ocean, Taiwan and the South Pacific, but have a broad gap in south-east Asia, western New Guinea and northern Australia. This and similar examples indicate that the New Caledonia – Madagascar direct track is probably related to another: New Caledonia – New Guinea – Madagascar/Mauritius, which skirts Australia to the north, e.g. the group Weinmannia/Pancheria/Cunonia (Cunon.). Likewise, the marine mollusc Microvoluta joloensis ranges in New Caledonia, Fiji (Lau)/Tonga, and Wallis & Futuna (with related fossil forms in New Zealand), and is disjunct in the Philippines and Madagascar (Bouchet & Kantor, 2003). 2. Tethyan tracks: New Caledonia – Mediterranean Invertebrates The lithistid sponge Siphonidium occurs in Florida, the Caribbean, the Azores, Western Africa, the Mediterranean, Indonesia, and New Caledonia (Manconi & Serusi, 2008). 3. Southern South Pacific track: Australia – New Zealand – New Caledonia – southern South America Notes on the main text Atherospermataceae (Laurales) are in eastern Australia/Tasmania (three genera), New Guinea (one genus), New Caledonia (one genus), and New Zealand-southern Chile (two genera). Fossil material is also known from the Kerguelen Plateau, the Antarctic Peninsula, Africa and Europe, but not America. The sister group is the family Gomortegaceae, with one genus in Chile (Heads, 2009c). In the beetle group Adeliini (see main text) none of the four major areas occupied is confined to a single part of the phylogenetic tree and ‘This suggests that diversification had already occurred before the first area separated’ (Matthews, 1998). Fossil-based phylogeography and other dispersal analyses would instead infer that this indicated dispersal, but Matthews’ suggestion seems more likely. In the Mesozoic, the fauna of the Murihiku terrane in New Zealand and faunas of equivalent terranes in New Caledonia and Argentina define a centre of endemism (Damborenea & Manceñido, 1992) which has persisted through the Cenozoic and is seen in many modern groups. Seed plants Libocedrus (Cupress.) of New Zealand and New Caledonia (fossil in south-eastern Australia) should include Pilgerodendron of southern Chile (Gadek et al., 2000). Nothofagaceae, the southern beeches, are in Australia, New Guinea, New Zealand, New Caledonia and southern South America, with fossil records also in Antarctica (Heads, 2006). In Proteaceae, a clade of seven genera form a mainly tropical track in eastern Australia – Melanesia – South America (Weston & Barker, 2006; Willis, 2007): 5 Hicksbeachia: eastern Australia, disjunct between Cairns area and the MMO (McPherson range to Coffs Harbour). Bleasdalea: north-eastern Australia, New Guinea. Sleumerodendron: New Caledonia. Kermadecia: New Caledonia. Turrillia: Vanuatu, Fiji. Gevuina: Chile, Argentina. Euplassa: widespread in tropical South America. Invertebrates Helicophidae (Trichoptera) are distributed in southern Australia, New Zealand, New Caledonia, Chile, and south-western Argentina (Johanson & Ward, 2001). Members of the family Peloridiidae (Hemiptera) are found among mosses and liverworts, usually in Nothofagus forest, and occur in Australia, Lord Howe I., New Zealand, New Caledonia, and southern South America (Evans, 1981). Although no fossils are known, Evans wrote that the group represented an ‘undoubted ancient lineage [which] must have been in existence before the breakup of Gondwanaland’. The species are ‘remarkably stable and though their present populations must have been isolated for very considerable periods of time they have developed no substantial structural differences’. In chrysomelid beetles, Spilopyrinae: Australia, New Caledonia, southern Chile, are sister to the world wide Eumolpinae (Gómez-Zurita et al., 2005). 4. Coral Sea (New Caledonia, north-eastern Queensland, Papuan Peninsula, Solomon Islands) Seed plants In Agathis (Araucar.) the three main clades are allopatric in New Zealand (basal), Fiji-Vanuatu, and Australia (Knapp et al., 2007), with the New Caledonian and Malesian species probably to be included with the last (Page, 1980). The tree Balanops, sole genus of Balanopaceae, ranges in Queensland (one species), New Caledonia (7 species), Vanuatu and Fiji (1). It is sister to a large pantropical complex (Trigoniaceae, Euphroniaceae, Dichapetalaceae, and Chrysobalanaceae; Stevens, 2009). Another basal group in the area is Melonycteris of the Bismarck Archipelago and Solomon Is., basal or near basal in fruit bats (widespread through the Old World) (Pulvers & Colgan, 2007). Other taxa based in and around the Coral Sea include the following. Piliocalyx (Myrt.; New Caledonia, Vanuatu, Fiji) is sister to a clade of Acmena restricted to Cairns/Cape York (Craven et al., 2006; the taxa were treated in Syzygium s. lat.). Santalum austrocaledonicum (Santal.) of New Caledonia and Vanuatu is sister to S. lanceolatum s. str. of Australia north of a line Broome – Townsville (Harbaugh & Baldwin, 2007; Harbaugh, 2007). Zygogynum (incl. Bubbia, Belliolum and Exospermum) (Winter.) is in north-eastern Australia, Lord Howe I., New Caledonia, the Solomon Is., and mainland PNG (Vink, 1985). Many Coral Sea groups have a massing in New Caledonia, for example, the family Myodocarpaceae, comprising the following three genera: Myodocarpus: New Caledonia. Pseudosciadium: New Caledonia. 6 Delarbrea: north-eastern Queensland, New Caledonia, Vanuatu, Solomon Is., New Britain, mainland PNG (Madang), Timor, and the Aru Is. (van Balgooy & Lowry, 1993; Plunkett, Wen & Lowry, 2004). The three genera were formerly associated with Araliaceae/Umbelliferae, but are now regarded as closer to Pittosporaceae, a widespread Old World family. Plunkett et al. (2004) wrote that radiation of Araliaceae is ‘possibly correlated with the breakup of Gondwanaland’, which included the rifting open of the Coral Sea following the opening of the Tasman Sea. Plant genera endemic here include the following. Euroschinus (Anacard.): Queensland and New South Wales (1 species), New Guinea and New Britain (1), New Caledonia (4) (Avé & van Balgooy, 1984). Storckiella (Caesalp.): Queensland (1 species), New Caledonia (3), and Fiji (1). Archidendropsis (Mimos.): Queensland (3 species), northern New Guinea (1), Solomon Is. (1), New Britain (1), and New Caledonia (8) (van Balgooy & Nielsen, 1993). Tapeinosperma (Myrsin.): eastern Australia (2 species), Solomon Is./ Bismarck Archipelago (4), New Caledonia (40), Vanuatu (2), Fiji (12), Tonga (1) (not in New Guinea) (van Balgooy, 1993d). Invertebrates Related species of Dasybasis (Diptera: Tabanidae) form a series surrounding the Coral Sea, in eastern Australia, PNG, Bismarck Archipelago, Solomon Is. (including Santa Cruz Is.), and New Caledonia (Mackerras, 1970). 5. Tasman Basin Lower plants Groups restricted to New Zealand – Tasmania with a northern outlier at New Caledonia include the liverworts Adelanthus bisetulus: Tasmania/Victoria, New Zealand (including the subantarctic Auckland and Campbell Islands) and New Caledonia, Acrochila: Tasmania, New Zealand, New Caledonia, Tetracymbaliella, the same range plus Campbell I. (Schuster, 1979, 1982), and Cephaloziella exiliflora: Tasmania, New Zealand, and New Caledonia. In the fern Gleichenia/Stromatopteris, a New Zealand, Tasmania, New Caledonia clade was retrieved in the sample of species studied by Perrie et al. (2007). Seed plants In Atherospermataceae, Nemuaron of New Caledonia is sister to Atherosperma distributed from north-eastern New South Wales to Tasmania (Renner et al., 2000). Some groups have most of their species around the Tasman Sea but also occupy north-eastern Queensland. For example, Dracophyllum (Eric.): eastern Australia (Tasmania to north-eastern Queensland), New Caledonia, Lord Howe I., New Zealand including the Subantarctic islands (most species) (van Balgooy, 1966a, Heads, 2009c). Hedycarya (Monim.) is in eastern Australia (Victoria to north-eastern Queensland; not in Tasmania) (2 species), New Caledonia (8 species), Vanuatu, Fiji/Tonga/Samoa (1) and New Zealand (1) (van Balgooy, 1993c). Invertebrates In Diptera, Chrysopilus tasmaniensis (Rhagionidae) is in New Caledonia and Tasmania. 7 In beetles, Percosoma (Carabidae) comprises two species in Tasmania and one in New Caledonia (Roig-Juñent, 2000) and belongs to a subtribe that is also in New Zealand (Mecodema etc.), Australia and South America. Other subtribes are in other parts of the world and as RoigJuñent (2000) concluded: ‘From a biogeographical point of view, the idea of either an austral or Holarctic dispersal center of origin is not supported. The tribe was probably distributed along the Pacific margin in the Jurassic’. Groups extending further north in Australia include the basal clade in the world-wide Micropterigidae (basal Lepidoptera). The clade comprises 11 species in Tasmania and along the east coast of Australia (north to Daintree near Cairns), two species in New Caledonia, and one in northern North Island, New Zealand (Gibbs, 1990, 2006; G. Gibbs pers. comm. 7 – viii – 2007). The spider Migas (Migidae) is in New Zealand (most species), Tasmania, Queensland, and New Caledonia. 6. New Caledonia – Australia Seed plants Canarium sect. Canariellum (Burser.) is in New Caledonia and Queensland: Townsville – Brisbane (Leenhouts, 1955). Archirhodomyrtus (Myrt.), formerly regarded as endemic to New Caledonia is now taken to include A.(Rhodomyrtus) beckleri of north-eastern New South Wales – north-easterrn Queensland by (Scott, 1978). Taxa with distributions that are similar but more widespread in eastern Australia include the snapper Lutjanus adetii which occurs along the New South Wales/Queensland coast and in New Caledonia (Allen, 1985) and the tree Sannantha (Myrt.) of Queensland – Victoria (10 species) and New Caledonia (4 species) (Wilson et al., 2007). Alectryon (Sapind.) ranges from south-east Asia to Australia and through the Pacific islands to Hawaii. New Caledonia has one (endemic) species. Edwards & Gadek (2001) accepted a ‘Gondwanan origin’ of the genus, but interpreted New Zealand and New Guinea members as the result of dispersal and presumably the New Caledonian species (not included in their study) would also be interpreted as due to dispersal. The only evidence given for dispersal was the cladogram topology and this can be interpreted instead as representing a sequence of vicariant events in a widespread ancestor. ‘All Australia’/southern New Caledonia distribution is seen in the clade Actinostrobus/ Callitris/Neocallitropsis (Cupress.) (Gadek et al., 2000). For Araucariaceae, Givnish & Renner (2004) began by inferring an Australian origin followed by ‘expansions’ into other islands of the south-west Pacific, including ‘at least two colonizations of New Caledonia by Agathis and Araucaria’. But after considering other paleobotanical and ecological data, Givnish & Renner concluded that ‘it is impossible to infer whether Agathis arose in Australia, Malesia, New Caledonia or any combination of these regions… Additional detailed paleobotanical data from Malesia and New Caledonia will be needed to resolve this issue.’ Another branch of Myrtaceae is the Melaleuca group of New Caledonia, Australia, New Guinea, and neighbouring areas. The New Caledonian taxa again form a clade that may be basal, ‘representing a relatively old vicariance event as for the eucalypt group’ (Ladiges et al., 2003). Bartish et al. (2005), Swenson, Bartish & Munzinger (2007), and Swenson, Munzinger & Bartish, 2007) found several clades of Sapotaceae each comprising a single Australian species sister to a relatively large group of New Caledonian endemics, ‘suggesting multiple dispersal events’ between the two countries. Bartish et al. (2005) wrote that ‘the phylogeny suggests an 8 interesting case of a relatively recent and rapid radiation of several lineages of Sapotaceae within New Caledonia’. The basis for their molecular clock calibration of the molecular dating was not mentioned, but was based on dates in Richardson et al. (2001). Richardson et al., in turn, did not mention the basis for the calibration and the rates were again derived from another paper. With respect to the Australia-New Caledonia Sapotaceae, repetitions of a particular disjunction, as in this case, can usually be interpreted as evidence for vicariance. For instance, discussing a disjunction between Tennessee and Arkansas in a Carex species, Naczi et al. (1998) concluded that ‘The repeated examples of this distribution pattern suggest that the disjunction of C. willdenowii in the Ouachita Mountains is not due to natural long-distance dispersal nor to introduction by humans’. With respect to the bird family Aegothelidae and its proposed centre of origin (see main text), Dumbacher et al. (2003) wrote that the New Caledonia and New Zealand species could have ‘arrived at any time on their respective landmasses’. The basal position of the New Caledonia-New Zealand pair in the phylogeny ‘raises the question of whether owlet-nightjars dispersed to these islands at a time when they were closer to Australia’. In fact there is no need for any ‘arrival’ or ‘dispersal’ across the Tasman Sea either eastwards or westwards; eastern and western clades probably evolved in the regions they currently occupy. Lower plants In species of Megalosporaceae (lichens), the tuberculosa-type of spore occurs in Australian species but is absent from Lord Howe, New Zealand and New Caledonia, while atrorubicans- and sulphurata-types show the reverse situation. There is a single exception for each spore type (Sipman, 1983). Invertebrates An example from the fauna of a diverse Australian group absent from New Caledonia is the gastropod family Bithyniidae (Ponder, 2003). The diving beetle genus Papuadytes has 20 species in Australia, 150 in New Guinea, and 30 in New Caledonia, but is not in Fiji, whereas the related Copelatus has related extensively in Fiji, but is not in New Caledonia. For Balke et al. (2007a) this indicates that ‘whether or not a lineage is on an island is due to chance’, but instead it is a simple case of vicariance between Australia/New Guinea/New Caledonia on one hand and Fiji on the other. Balke et al. (2007a) recovered a phylogeny for Papuadytes: (Australia (New Caledonia (Australia (Australia (New Guinea (China (New Caledonia, Australia))))))). They wrote that the New Caledonian fauna is composed of two clades each with a sister group in Australia. But their cladogram indicates that the more basal New Caledonian clade is sister to a large group of Australia, New Guinea, China and New Caledonia, indicating an early divergence and subsequent overlap. The authors suggested that dispersal has played an important role in the history of Papuadytes, but the molecular clock dates were the only evidence for this. The clock was calibrated on the basis that central Australian taxa diverged at 10 Ma. This date was based on a date for other central Australian beetles living in the same locality and the same groundwater habitat. The basis for this date was not given. In any case, this procedure of ‘ecological cross calibration’ is very prone to error as taxa often persist in a changed habitat. In flies, the predominantly Australian genus Australophlebotomus (Psychodidae) has a ‘derived’ species pair in New Caledonia that could be the result of Paleocene vicariance (Léger & Depaquit, 2002, as cited by Cranston, 2005). Another fly genus, Antyx (Dolichopodidae), is known from the 9 Blue Mountains (by Sydney), the McPherson-Macleay Overlap (see section 7), north-eastern Queensland, and New Caledonia (Bickel, 1999). Bickel suggested a ‘minimum late Cretacous age’ and ‘vicariant Gondwanan’ origin for the genus. 7. New Caledonia – McPherson-Macleay Overlap (MMO)/north-eastern Queensland Notes on the main text The MMO has been recognised as significant by many biogeographers, such as Croizat (1964) and Holloway (1979). For Melicope and relatives (see main text), Poon et al. (2007) found that Melicope vitiflora from Fiji grouped with Pitaviaster. but did not sample the New Caledonian plants. Direct New Caledonia – north-eastern Queensland connections are exemplified by the genus Austrovelia (Hemiptera; Andersen & Polhemus, 2003). A connection between New Caledonia and Cape York Peninsula in northern Queensland occurs in the spider Argiope lobata (Levi, 1983) and the Bledius circularis group of staphylinid beetles (Herman, 1986). Lower plants The lichen Pseudocyphellaria argyracea occurs around the Indian Ocean, and from New Zealand, New Caledonia and New Guinea east through the Pacific, but is in Australia only at the MMO (Galloway, 1994). The liverwort Acrolejeunea securifolia has one subspecies endemic in New Caledonia, one in the Philippines, New Guinea, northern Solomon Is.; one at the MMO, Norfolk I., north-eastern New Zealand; and one in the Cook Is. and French Polynesia (Gradstein, 1975). The moss Thuidium protensulum is in New South Wales/Queensland, Lord Howe I., and New Caledonia. Seed plants In Hernandia subgen. Hernandia (Hernand.; Kubitzki, 1970), the ‘moerenhoutiana Group’ of New Caledonia, Vanuatu, the Solomon Is., and east to the Society Is. and central America/West Indies is vicariant with the ‘bivalvis Group’, endemic to the MMO. The Cunoniaceae are especially diverse in New Caledonia. Vesselowskya is endemic to the MMO (between the Hunter and Clarence Rivers in north-eastern New South Wales) and is sister to a clade Pancheria (New Caledonia), Cunonia (New Caledonia, South Africa) and Weinmannia (Mascarenes, Malesia, New Zealand, Pacific islands, South America) (Hufford & Dickison, 1992; Bradford, 2002). Some New Caledonia – MMO groups also extend north in Australia. Neorapinia collina (Labiatae) of Norfolk I., New Caledonia and southern Vanuatu forms a group (Neorapinia) with Vitex lignum-vitae of the MMO/north-eastern Queensland and V. lucens of northern New Zealand (Mabberley, 1998). In a related pattern, Duboisia (Solan.) comprises four more or less vicariant species: D. arenitensis in northern Australia (Arnhem Land), D. hopwoodii in central and western Australia, D. leichhardtii inland by the MMO, and D. myoporoides in coastal eastern Australia (QueenslandNew South Wales) and New Caledonia (Haegi & Symon, 1984). Geissois s. lat. (Cunon.) is at the MMO, disjunct in north-eastern Queensland, also (Geissois s. str.) in New Caledonia (centre of diversity), Vanuatu, and Fiji (Hoogland, 1984; Hopkins, 2006). Xylosma (Flacourt.) is in south-east Asia (5 species), Malesia (4 species), MMO (two locally endemic species; Jessup, 1982), New Caledonia (20 species), and South America (c. 70 species). 10 Canavalia sericea (Legum.) ranges from the Caroline Is. to Fiji/Tonga/Samoa and east to the Society Is., with south-western outliers at New Caledonia (I. des Pins) and between the MMO and north-eastern Queensland (Sauer, 1964). In Trimenia (Trimen.), the single New Caledonian species is a tree, as are the species of Sulawesi, New Guinea, Solomon Is., and Fiji to the Marquesas, but through its pollen the New Caledonian species has affinities with the high-climbing liane species, known from the MMO, north-eastern Queensland and PNG (Philipson, 1986). Invertebrates The mesogastropod Pseudopisinna gragaria rigifera is in New Caledonia and at the MMO (Ponder & Yoo, 1980). The cowry Nesiocypraea langfordi moretonensis is in New Caledonia and at the MMO, and the other subspecies in the species, N. l. langfordi, is disjunct at the Philippines/southern Japan (Lorenz & Hubert, 1993) (see below for this disjunction). The lobster Parribacus caledonicus follows a linear distribution: MMO, New Caledonia, Vanuatu, Fiji, and Samoa (Holthuis, 1991). Vertebrates The angelfish Centropyge flavissima is at Christmas and Cocos-Keeling Is. in the eastern Indian Ocean, Micronesia to eastern Polynesia, Melanesia and New Caledonia with an outlier at the MMO (Allen, Steene & Allen, 1998). 8. New Caledonia – Lord Howe I./Norfolk I. Notes on the main text Page et al. (2005) assumed that Paratya is able to undertake long-distance trans-oceanic dispersal, but only because it occurs on isolated oceanic islands, and they cited endemic Paratya species on the Bonin Is. and the Chatham Is. east of New Zealand as support for this. The Bonin Is. occur at a subduction zone (the Mariana Trench) where islands have been coming and going since long before the modern islands existed. The Chatham Is. include Mesozoic terranes of the New Zealand basement and have a record of persistent volcanism. The biota includes many old New Zealand taxa. It is suggested that they also preserve an old, central Pacific biota transported on the former islands (now seamounts) of the Hikurangi Plateau to the Chatham subduction zone in the Cretaceous (Heads, 2009b). Page et al. (2005) proposed that ‘The first biogeographical issue to consider is the geographical origin of the genus Paratya’, but there is no need to assume that there was a centre of origin located within the current range of the genus, and that the other species somehow migrated out from here. There is no evidence for this and it seems improbable given the precise mosaic patterns of endemism and vicariance in the genus. The (New Caledonia (Norfolk + Lord Howe)) arrangement is just one of these. Keast (1966) cited relationships of the New Caledonia biota with Norfolk and Lord Howe Is. and also Australia. He described the mist-shrouded summit plateau of Lord Howe I. with dense moss forest and relict taxa such as the hemipteran family Peloridiidae (see track 3, above). Lillemets & Wilson (2002) described the ‘surprising’ diversity of terrestrial isopods on Lord Howe. They interpreted the fauna and the land as ‘contracted remnants’, and the taxa (including poorly dispersing groups) as ‘ancient relicts’ of a formerly more widespread biota. Australiodillo is one example, being endemic to Lord Howe (five species), New Caledonia (two species) and 11 Queensland/New South Wales (one species) (see notes on New Caledonian isopods at the end of the Appendix). Taxa endemic to Lord Howe and Norfolk Is. include the fern Cephalomanes bauerianum, the seed plants Elymus multifllurus var. kingianus (Gram.), Dianella intermedia (Herocallid.), Lagunaria patersonia subsp. patersonia (Malv.), Solanum bauerianum (Solan.) and Calystegia affinis (Convolvul.) (all from Green, 1994), and fauna such as the anemone fish Amphiprion mccullochi (Fautin & Allen, 1992). Lower plants The fern Doodia media subsp. media is in eastern Australia, Lord Howe, Norfolk and New Caledonia, and plants from the last three localities are most similar (Green, 1994). Seed plants Taxa connecting the Lord Howe/Norfolk centre with New Caledonia include Korthalsella taenioides f. emersa (Visc.) on Lord Howe, Norfolk, and New Caledonia (Molvray, 1997) and Oberonia titania (Orchid.) on Lord Howe, Norfolk, New Caledonia and Vanuatu (Lewis & Cribb 1989). Tylophora biglandulosa (Asclepiad.) has the same range plus Fiji (Green, 1994). The shorefish Plesiops insularis is at Lord Howe, Norfolk, New Caledonia and the Is. Chesterfield (north-west of New Caledonia) (Mooi, 1995). Taxa with eastern Australia (especially MMO) – New Caledonia connections via Lord Howe and Norfolk Is. include the orchid Bulbophyllum argyropus (Orchid.) of the MMO, Lord Howe and Norfolk, very closely related to B. corythium of New Caledonia (Green, 1994). Celtis paniculata (Ulm.) is widespread in coastal eastern Australia, Lord Howe, Norfolk, and New Caledonia (Green, 1994). In Sarcomelicope (Rut.) there are five species endemic on New Caledonia and one other. This has one subspecies in Queensland/New South Wales, Lord Howe and Norfolk Is., another in New Caledonia and Vanuatu, and a third in Fiji (Hartley, 1982). Elaeodendron australe (Celastr.) of eastern Australia is related to E. curtipendulum of Lord Howe, Norfolk and New Caledonia (Green, 1994). An interesting arc south of New Caledonia is displayed by Zanthoxylum pinnatum (Rut.): Lord Howe, Norfolk, southern Vanuatu (Erromango), Fiji/Tonga, with the closely related Z. pancheri on New Caledonia (Green, 1970). Invertebrates The beetle genus Dematochroma (Chrysomelidae) has most species in New Caledonia, but has two endemics on Lord Howe and two on Norfolk (and one on Timor) (Jolivet et al., 2007a,b). The genus is absent from Australia and New Zealand but may possibly occur in other parts of Melanesia. Vertebrates The spectacular angelfish Chaetodontoplus conspicillatus occurs from the Great Barrier Reef south to central New South Wales, and also on reefs around Lord Howe I., Norfolk I., and New Caledonia (Allen et al., 1998). Similar connections are seen in other reef fishes, such as the tripterygiid Enneapterygius rufopileus of Lord Howe I., Norfolk I., New Caledonia, and Fiji/Tonga (Fricke, 2002) and the gobiid Eviota hoesi endemic to Lord Howe I., reefs on the Lord Howe Rise (Middleton and Elizabeth Reefs), Norfolk I., and New Caledonia (Gill & Jewett, 2004). 12 9. New Caledonia – Lord Howe Island Lower plants In lichens, Megalospora sulphureorufa is in Lord Howe and New Caledonia (southern Grande Terre, I. des Pins, and the Loyalty Is.) (Fig. 5; Sipman, 1983). The mosses Ctenidium pubescens, Entodon pancherianus and Fissidens arcuatus, and the fern Blechnum contiguum are known only from Lord Howe and New Caledonia. Seed plants Pandorea pandorana subsp. austrocaledonica (Bignon.) is on Lord Howe, New Caledonia and southern Vanuatu (Aneityum) (Green, 1994), Rottboellia coelorachis (Gram.) is also on Lord Howe, New Caledonia and southern Vanuatu (Tanna and Erromango) (Green, 1990). Zygogynum (incl. Bubbia, Belliolum and Exospermum) (Winter.) ranges in Lord Howe I., New Caledonia, the Solomon Is., PNG, and north-eastern Australia (Vink, 1985). The Guioa microsepala clade (Sapind.) occurs in Australia, Lord Howe and New Caledonia (van Balgooy et al., 1996). Polyscias cissodendron (Aral.) is on Lord Howe, New Caledonia, Vanuatu, and the Solomon Is. (Eibl et al., 2001). Invertebrates The spider Argyrodes gracilis (Theridiidae) is only on Lord Howe, New Caledonia, and Samoa. The deep sea crustacean Kimbla comprises one species of New Caledonia and one from Taupo Seamount, south-west of Lord Howe I. (E 156° 10’, S 33° 15’) (Richer de Forges, 1993). Vertebrates In morwongs (perciform fishes), Cheilodactylus francisi occurs around Lord Howe, New Caledonia, the Kermadec Is. and Hawaii (Burridge & Smolenski, 2004). The rail genus Tricholimnas (= Gallirallus lafresnayanus) is only on Lord Howe and New Caledonia. 10. New Caledonia – Norfolk Island The islands along the Norfolk Ridge have high terrestrial endemism and the marine fauna on the seamounts along the ridge also show high diversity and endemism (Richer de Forges, Koslow & Poore, 2000). Holloway (1996) wrote that ‘Evidence for the geological youth of Norfolk Island and its isolation since emergence through the volcanic events commencing 3 million years ago appears to be strong… and may be sufficient to justify the assumption that the island acquired its biotas by dispersal from the three nearest source areas, Australia, New Zealand and New Caledonia’. But this approach of relying solely on current geographic areas overlooks the possibility that there may have been other land in the region, as was later shown in this case. Lower plants The liverworts Anthoceros elegans and Radula robinsonii are both known only from New Caledonia and Norfolk. Plagiochila norfolkiensis has the same range, and So & Grolle (2001) compared it with a New Guinea endemic. P. inflata is in New Guinea, New Caledonia and Norfolk (So, 2000). 13 Seed plants The thirteen New Caledonian Araucaria species form a clade that is sister to the Norfolk species A. heterophylla (Setoguchi et al., 1998). Euphorbia norfolkiana (Euphorb.) of Norfolk is very close to E. kanalensis of New Caledonia (Green, 1994). Exocarpus phyllanthoides (Santal.), Korthalsella disticha (Visc.) and Zehneria baueriana (Cucurbit.) are all endemic to New Caledonia and Norfolk I. (Green, 1979, 1994). Dysoxylum bijugum (Mel.), Euphorbia obliqua (Euphorb.), Tropidia viridifusca (Orchid.) and Phreatia paleata (Orchid.) are all on Norfolk I., New Caledonia and Vanuatu (Mabberley, 1998; Green, 1994; Lewis & Cribb, 1989). Invertebrates In papilionid butterflies, Papilio ilioneus is on New Caledonia and Norfolk, Anaphaeis java peristhene is at these localities plus Vanuatu (Holloway & Peters, 1976). In other Lepidoptera, Holloway (1993) cited Norfolk – New Caledonia sister group relationships in Hydrillodes (related next to Fiji/Tonga, Kermadec Is.), Pyrrhorachis (next to Vanuatu), Agathia (next to New Guinea and Fiji), Ericeia (next to Vanuatu), Thalassodes (next to New Guinea and Bismarck Archipelago) and Cleora (New Caledonia/Vanuatu sister to Norfolk, then this group sister to Society and Rapa I.). In addition, Trigonistis toroensis (Noctuidae) of New Caledonia is sister to T. andersoni of Norfolk, and Luceria cooki (Noctuidae) is restricted to Norfolk and New Caledonia, Trissernis greeni (Noctuidae) is on Norfolk, New Caledonia and Vanuatu, and Ericeia hirsutitarsus (Noctuidae) is on Norfolk, New Caledonia, the Loyalty Is. and Vanuatu (Holloway, 1979). 11. New Caledonia – New Zealand Notes on the main text Iredale (1912, p. 81) commented that the New Zealand – New Caledonia connection ‘has been constantly overlooked by most New Zealand writers’. Chambers et al. (2001) interpreted the New Caledonia – New Zealand connection as an indication of dispersal to or from New Caledonia, but the molecular clock calibrations that they based their conclusions on are not accepted here. New Zealand – New Caledonia distribution is probably not due to dispersal from one country to the other (either prior to geological separation or after separation), but could be due to the vicariance of a widespread ancestor which also gave rise to Australian or Pacific relatives. Based on the 3 Ma date as a maximum age for the Kermadec Is. and Norfolk species, Buckley & Simon (2007) inevitably concluded that Maoricicada (and the rest of the New Zealand alpine biota) is ‘very young’. (But even with calibration points which are too young, a Miocene age for the group was calculated and this is before the main rise of the New Zealand Southern Alps. Buckley & Simon (2007) referred to ancestral Maoricicada as being ‘preadapted’ to the alpine habitat and this would make sense as the group has survived uplift so successfully while maintaining largely allopatric distributions. It also indicates that the age of the mountains cannot be used to calibrate the age of taxa endemic there). The basal position of the New Caledonian members in Cyanoramphus and the ideas of Herzer et al. (1997) on a ‘landbridge’ between New Caledonia and New Zealand led Boon et al. (2001) to propose an Australian origin for Cyanoramphus, followed by dispersal to New Caledonia and then to New Zealand. They suggested that the New Zealand – New Caledonia dispersal occurred within the last 500 000 years, assuming a molecular clock and rates inferred for another bird group (the 14 basis for this rate was not given). This age is much younger than the proposed land bridge, and so the ‘land bridge theory of colonisation… may not be a viable explanation’ (Boon et al., 2001). Former islands such as the one described from near Norfolk may have supplied both southern New Caledonia and northern New Zealand with biota as they came into existence; southern New Caledonia + northern New Zealand (Poor Knights/Hen and Chickens Is.) is a centre of endemism for groups such as the monocot family Xeronemataceae (Heads, 2009a). Lower plants In liverworts, Goebeliella is monotypic in New Zealand (the subantarctic Auckland Islands, the main islands and the Chathams) and New Caledonia, and is sister to a large, more or less cosmopolitan group: Radulaceae, Frullaniaceae, Jubulaceae plus Lejeuneaceae (Heads, 2009a). Schuster (1969) referred to a ‘fair number’ of liverwort species with the New Caledonia – New Zealand distribution. Miller et al. (1983) cited Chiloscyphus physanthus, Marchantia pileata, Riccardia plana, Schusterella microscopica, Tetracymbaliella comptonii and Zoopsis ceratophylla from New Caledonia and New Zealand only, Frullania squarrosula, Radula dentifolia and Riccardia bistrata from New Caledonia and North I., and Geocalyx caledonica from New Caledonia, South I. and Stewart I. Lopholejeunea colensoi is in New Caledonia and Campbell I. Schistochila caledonica is restricted to New Caledonia and the Paparoa Range, an important biogeographic centre in the South Island (So, 2003). In mosses, Eucamptodon inflatus is restricted to New Caledonia and New Zealand. The pteridophyte Tmesipteris lanceolata is endemic to New Zealand and New Caledonia (Chinnock, 2003). The New Caledonian fern Adiantum novae-caledoniae is closely related to A. fulvum of New Zealand and Norfolk I. Molecular studies showed that Hymenophyllum paniense of New Caledonia is closest to H. scabrum of New Zealand (Ebihara et al., 2003). Seed plants In podocarp trees, Philippi in Aubréville et al. (1967-) wrote that Prumnopitys ferruginoides (Podocarp.) of New Caledonia has a conspecific form in New Zealand, but this actually refers to the closely related ‘corresponding species’ in New Zealand, P. ferruginea (de Laubenfels, 1978, D. de Laubenfels, pers. comm. 15 – v – 2008). These podocarps are the largest trees in many forests and their New Caledonia – New Zealand affinity is of special interest. The monocots Astelia sect. Isoneuron (Astel.; Skottsberg, 1937) and Xeronema (above) are both only known from northern New Zealand and New Caledonia. The New Zealand endemic orchid Danhatchia (northern North Island, north-western South Island) is closely related to the New Caledonian Gonatostylis (Ormerod & Cribb, 2003a). Corynocarpus laevigatus (Corynocarp.) of northern New Zealand (including the Chatham and Kermadec Is.) is sister to C. dissimilis of New Caledonia (Wagstaff & Dawson, 2000). The New Caledonian dicot Strasburgeria is sister to the New Zealand Ixerba (Strasburger.). Schefflera sect. Schefflera (Aral.) is in New Zealand, New Caledonia, Vanuatu, and Fiji/Samoa (Plunkett et al., 2005) (cf. the liverwort Schusterella, in New Zealand, New Caledonia and Fiji). The angiosperm Gymnostoma (Casuarin.) has a wide South Pacific distribution in Malesia, north-eastern Queensland, the Solomon Is., New Caledonia and Fiji, and, fossil, in the rest of eastern Australia, New Zealand, and South America. The earliest known fossils are Paleocene. In the species they sampled, Steane, Wilson & Hill (2003) found two clades, one in Malesia/Australia (six species) and one in New Caledonia (four species), and suggested that long-distance dispersal from New Zealand to New Caledonia might explain the New Caledonian presence of 15 Gymnostoma. They reached this conclusion on the basis of fossil-based dates and proposed as ‘significant’ the fact that ‘while the Casuarinaceae have a fossil record in New Zealand that dates back to Paleocene … it does not extend back to the time when New Zealand is believed to have separated from Gondwanaland’. They concluded that this ‘suggests a requirement for dispersal… or a poorly known fossil record’. As the second alternative is very likely, the first is probably unnecessary. In Proteaceae, Garnieria of New Caledonia (southern and north-western Grande Terre) is sister to Toronia of New Zealand (North Cape to East Cape), and the unrelated Knightia comprises two species in New Caledonia and one in northern New Zealand (Weston & Barker, 2006). The plants Phelline (Phellin.), Paracryphia (Paracryph.) and Amphorogyne (Santal.) were previously only known from New Caledonia but fossil leaf material has been described from southern New Zealand (Pole, 2009). Invertebrates The long history of the New Zealand – New Caledonia region as a biogeographic centre is indicated by many Mesozoic fossil groups endemic to it, for example, the gastropod Protodolium, known only from the Cretaceous of New Zealand, the Chatham Is., and New Caledonia (Stilwell, 1994). The bivalve mollusc Monotis is one of the most characteristic taxa of the Upper Triassic. Of the five subgenera (Grant-Mackie, 1978), Monotis subgen. Inflatomonotis is in New Zealand, New Caledonia, and British Columbia, and M. subgen. Maorimonotis is restricted to New Zealand and New Caledonia. Overall, invertebrate faunas of the New Zealand and New Caledonian Triassic show ‘remarkable similarity’ (Campbell, 1994), with many taxa only known from the two localities (Campbell & Grant-Mackie, 1995; Begg & Grant-Mackie, 2003). In molluscs, Athoracophoridae are a family of terrestrial slugs endemic to the lands and islands around both Coral Sea and the Tasman Sea (see section 5). They have most of their species in New Zealand (including the Subantarctic and Chatham Islands; Burton, 1963, 1980) and New Caledonia; others occur in Vanuatu, the Solomon Is., the Bismarck Archipelago, Papua New Guinea mainland, and eastern Australia (north-eastern Queensland to Sydney; not in Tasmania) (Heads, 2009a). The sister group of Athoracophoridae is the cosmopolitan family Succineidae. The gastropods Alcithoe and Ataxocerithium are both known from New Zealand and New Caledonia, a distribution explained by the historically continuous distribution of the genera on the Norfolk Ridge (Bouchet & Kantor, 2003). In spiders, Cambridgea (Stiphidiidae) is only in New Zealand (including Chatham Is.) and New Caledonia, as is Desis marina (Desidae). Hypodrassodes (Gnaphosidae) is in New Zealand, Lord Howe, and New Caledonia. In beetles, Astetholea (Cerambycidae) is restricted to New Zealand and New Caledonia (Lu & Wang, 2005). In cerambycid beetles, tribe Enicodini is restricted to New Zealand and New Caledonia (Gressitt, 1961). In staphylinid beetles, the tribe Nesoneini has the same range, with the related tribes in Australia, New Zealand and southern South America (Silphotelini and Austrothysini), and Australia and New Zealand (Anepiini). This is, ‘consistent with an ancient origin and vicariance during breakup of Gondwana’ (Newton, 2004). In anthribid beetles, Androporus, Dasyanthribus, Helmoreus and Micranthribus are all restricted to New Caledonia and New Zealand (Holloway, 1982). 16 In Diptera, Anomalomyia, Tetragoneura, Sigmoleia (all Mycetophilidae), Aphopeas (Tabanidae) and Nervijuncta (Ditomyiidae) are all restricted to New Caledonia and New Zealand. Parentia (Dolichopodidae) is ‘a Gondwanan genus confined to Australia, New Zealand and New Caledonia’ in which the New Caledonian species appear to be close to New Zealand forms (Bickel, 2002). Also in Dolichopodidae, the distinctive xanthurus group of Paraclius comprises seven species in New Caledonia and one in North Island, New Zealand (Bickel, 2009). Vertebrates In birds, the snipe Coenocorypha (Scolopacidae) is extant on the New Zealand subantartctic islands (Snares, Auckland, Campbell, Antipodes and Chatham Is.), with fossil records on North and South Islands of New Zealand, Norfolk I., New Caledonia and Fiji (Worthy, Miskelly & Ching, 2002). The extinct rail genus Tricholimnas of New Caledonia and Lord Howe is closest to Capellirallus of North Island, Cabellus of Chatham Is., and Vitirallus of Fiji (all extinct; Worthy 2004). The bird Rhynochetos (Rhynochetidae) of New Caledonia is sister to the fossil Aptornis of New Zealand (Livezey, 1998). 12. New Caledonia – (Rennell I.) – New Guinea Notes on the main text Many groups show affinities between New Caledonia/Vanuatu and Papua New Guinea, especially northern PNG and the Bismarck Archipelago, but have no records from the Solomon Is. In some cases this will be due to lack of adequate collecting, but often there does seem to be a direct New Caledonia – New Guinea connection (sometimes with a Solomon Islands vicariant filling the gap). The New Caledonia – New Guinea track is documented in many scattered publications and in an earlier track analysis Morrone (2002) proposed New Caledonia – New Guinea as the ‘Neoguinean region’, one of just 12 for the world. The disjunction between the Vogelkop and the Bismarck Archipelago that occurs in Phyllanthus bourgeosii (Fig. 6) is also seen in forms of Osmoxylon (Aral.) and is related to the important connections between the Moluccas and New Britain, and between Waigeo and New Ireland (Heads, 2001, 2003: Fig. 73). At its three disjunct localities P. bourgeoisii grows on the edge of mountain torrents as a rheophyte. Webster and Airy Shaw (1971) described the distribution as ‘curious’. A New Caledonia and New Guinea disjunction also occurs in Phyllanthus pancherianus. Sampling of Zosterops species in the Moyle et al. (2009) study was not complete, but it is clear that Z. rennellianus is not directly allied with nearby species of the southern Solomon Is. or Vanuatu. In Phillimore’s (2006) sample of New Zealand, Lord Howe, Norfolk and Vanuatu populations, Z. rennellianus was linked with Z. tenuirostris of Norfolk I. The New Caledonia – Santa Cruz connection in the Pteropus pselaphon group (Fig. 7) also occurs in plants (Austrobuxus sp.; Picrodendr.; van Balgooy & Franken, 1984). (Politically the Santa Cruz group is in the Solomon Is., biogeographically it is allied closely with Vanuatu, Fiji and, as here, New Caledonia). A Solomon Is. – Moluccas connection similar to that of the chrysoproctus group occurs in Odonata (Rhinocypha libereta of Guadalcanal and its allies in the Moluccas; Polhemus et al., 2008) and in podocarps (Dacrydium magnum and Podocarpus spathoides; Heads, 2003: Figs. 80, 87). Lower plants 17 In fungi, Mycena irritans is in New Caledonia and New Guinea (Horak, 1983). In lichens, Pseudocyphellaria clathrata is known from Norfolk I., New Caledonia, New Guinea, western Malesia, Africa and South America (Galloway, 1994). The liverworts Plagiochilon theriotanus and P. braunianus are in New Caledonia, New Guinea, and further west in Malesia etc. (Schuster, 1979). Frullania heteromorpha, Jungermannia hirticalyx and Telaranea kogiana are all in New Caledonia and eastern New Guinea. Cololejeunea wightii is in New Caledonia, New Guinea, and Penang (Peninsular Malaysia). (The disjunction between New Guinea and Peninsular Malaysia seen in the last species is standard, cf. Heads, 2003). Marchantia lecardiana is in New Caledonia and New Britain, M. multiloba is in New Caledonia, New Guinea and the Philippines. Herbertus leratii is a rare endemic from southern Grande Terre, compared with a species of New Guinea/Indonesia by So (2003). Porella maxima is in New Caledonia and Papua New Guinea (So, 2002). Porella acutifolia has a similar connection: in the South Pacific and Australasia there are two varieties of this species, var. acutifolia in eastern and south-east Asia, New Guinea and Hawaii, and var. linguifolia of New Caledonia (So, 2002). Plagiochila inflata is in New Guinea, New Caledonia and Norfolk (So, 2000). Examples in mosses include Bescherellia elegantissima of New Caledonia, Loyalty Is., and the PNG Highlands (Sastre-De Jesús, 1987). Miller et al. (1978) recorded Calymperes tahitense var. denticulatum, Fissidens laxiretis, and Megalostylium papuanum all in New Caledonia and New Guinea. Hymenodon tenellus (also Goodenough I.) and Taxithelium nitidulum (also d’Entrecasteaux Is.) have similar ranges and Rhizogonium novaehollandiae var. minus is recorded from I. des Pins and New Guinea. Trematodon novae-caledoniae is in New Caledonia and the Bismarck Archipelago, T. hannoverae is in New Caledonia and New Hanover (off northern New Ireland). Spiridens vieillardii is in Lord Howe, New Caledonia and New Guinea. Leskeodon acuminatus is in New Caledonia, New Guinea, Java and Borneo. In ferns, Ctenopteris subsecundo-dissecta is restricted to New Guinea and New Caledonia. Schizaea wagneri of New Guinea and Malesia is closest to S. intermedia of New Caledonia (Selling, 1946). S. inopinata of Malesia east as far as western New Guinea and Micronesia is closest to S. laevigata of New Caledonia and S. melanesica of New Caledonia, Fiji and Tonga. Coryphopteris fasciculata (Thelypterid.) is in New Caledonia, New Guinea, and Sulawesi (Holttum, 1977). Seed plants The best-known plant group restricted to New Guinea and New Caledonia is Nothofagus subgen. Brassospora (Nothofag.). It comprises 19 species of large trees in New Caledonia and New Guinea and is the only Nothofagus subgenus present in these countries. Fossils of the group are known from New Zealand, Australia, Antarctica and South America. The extant range of Brassospora may represent the original centre of diversity for the group which has become extinct in secondary outliers (Heads, 2006). The other subgenera have their respective cores in different localities, and so probably all formed as vicariants. This explains the notable absence of the New Zealand and Australian subgenera from the mountains of New Guinea and New Caledonia. Direct New Caledonia – New Guinea connections, not involving the Solomon Is. or Vanuatu, also occur in the following taxa. In monocots, Sciaphila densiflora (Triurid.) occurs in New Caledonia, New Guinea (including Milne Bay islands), Malesia and Sri Lanka. S. corallophyton is in New Caledonia, north-eastern New Guinea, and eastern Caroline Is. (Ponape) (van Meerendonk, 1984). 18 The sea grass Enhalus (Hydrocharit.) is in New Caledonia, New Guinea and Torres Strait, and further west (den Hartog, 1970). Rhuacophila (previously treated in Dianella) (Hemerocallid.) is in Malesia, New Guinea, New Caledonia and Fiji (Clifford, Henderson & Conran, 1998). In dicotyledons, Nepenthes (Nepenth.) has closely related forms in New Caledonia (N. vieillardii) and western New Guinea (N. lamii; Cheek & Jebb, 2001). Alyxia (Apocyn.) is widespread from India to south-eastern Polynesia but has its major centres of diversity in New Guinea and New Caledonia (Avé, 1984). Ser. Laxiflorae comprises A. leucogyne of New Caledonia and species of New Guinea, Sulawesi, and the Philippines (Markgraf, 1977). Dicarpellum (Hippocrat.) of New Caledonia is closest to Brassiantha of New Guinea and Sarawakodendron of Borneo (Aubréville et al., 1967-). Coode (1987) proposed that Dubouzetia (Elaeocarp.) of New Caledonia, New Guinea, and the Northern Territory forms a group with Peripentadenia of north-eastern Queensland and Crinodendron of temperate South America, with all three showing clear vicariance. Dubouzetia comprises three sections (Coode, 1987): sect. Spongosperma: New Guinea, sect. Dubouzetia: New Caledonia, sect. Oligovula: New Guinea/Moluccas, the Northern Territory (Australia), and New Caledonia. For Coode this represents a ‘peculiar picture’ as it is not compatible with ‘island-hopping’ along the Melanesian arc – ‘The basic problem seems to be that D. elegans (sect. Oligovula) is found on both New Caledonia and the New Guinea mainland (but not the New Hebrides, Solomon Is. or Bismarck Archipelago [or Australia])’. Coode thought that ‘perhaps it is a New Guinea species which reached New Caledonia relatively late…’, but the distribution of the sections instead indicates that the New Guinea – New Caledonia sector is fundamental in the genus as a whole and not a secondary development. Hunga (Chrysobalan.) is restricted to New Caledonia (five species) and PNG (three species) (Prance, 1979). In PNG the genus occurs in the Papuan Peninsula (the ‘tail’ of the country) on and east of a line: Bakaia/Sogeri, and also in the Louisiade Islands (Milne Bay) with H. longifolia, endemic to Misima I. Prance regarded H. longifolia as most closely related to H. lifouana of New Caledonia, not to the other PNG species. This pattern is repeated in Loranthaceae: an ‘extreme variant’ of the south-west Pacific Amyema artensis occurs on Rossel I. (Louisiade Is.) and New Caledonia (Barlow, 1992). A. scandens is only known from New Guinea and New Caledonia. Barlow suggested that both A. artensis and A. scandens reached New Caledonia by dispersal from New Guinea, but this does not explain the absence from the Solomon Is., Vanuatu, and Australia. The closest relative of Hooglandia (Cunon.) from New Caledonia (north-eastern Grande Terre) ‘may prove to be Aistopetalum’, a New Guinea genus (McPherson & Lowry, 2004). Sloanea sect. Antholoma (Elaeocarp.) (van Balgooy, 1971) is restricted to New Caledonia and New Guinea. Thorne (1965) cited Pelma (Orchid.) as a New Guinea – New Caledonia group. In Aubréville et al. (1967-) Pelma is synonymised under Bulbophyllum, but its only New Caledonian species, B. (P.) neocaledonicum, is described as related to ‘Indonesian’ species and so the New Caledonia – New Guinea affinity may remain. In Myrtaceae, Xanthomyrtus occurs in New Caledonia, southern New Ireland, New Guinea (most species), Borneo and the Philippines. It may be significant that Scott (1979a) keyed out the only New Caledonian species, X. hienghenensis, next to X. schlechteri, widespread through New 19 Guinea to Fergusson I. and New Ireland. Uromyrtus artensis of New Caledonia may be closest to U. novoguineensis of the Cyclops Mts. (above Jayapura, north central New Guinea) (Scott, 1979b). In groups with New Caledonia – Bismarck Archipelago connections, Heliconia indica var. austrocaledonica (Helicon.), of southern New Caledonia and western Vanuatu, keys out with H. i. micholitzii of New Ireland and Manus, rather than with H. i. denisiana of the Solomon Is. or H. i. rubricarpa of north-eastern PNG and New Britain (Kress, 1990). Meiogyne glabra (Annon.) of central New Britain is keyed out next to M. ‘spec. 1’ of New Caledonia by van Heusden (1994). (Glabrous forms found around Suva, Fiji, may also belong here; pers. obs. 2005). Arthrophyllum (Aral.) occurs in New Caledonia, the Bismarck Archipelago, New Guinea, and through Malesia to south-east Asia (Philipson, 1979; van Balgooy, 1993a; Frodin, 2001). Dolichandrone spathacea (Bignon.) has a similar distribution but extends to Sri Lanka and India (van Steenis, 1963). Invertebrates Many species of coral are in New Caledonia and New Guinea (and other areas) but do not occur in Australia (Veron, 2000), despite the presence of suitable habitat on the Great Barrier Reef. The gastropod Xenophora granulosa is in New Caledonia, New Britain, the Philippines and Mauritius (Ponder, 1983). In lobsters, Ibacus brevipes is in New Caledonia, south-western New Guinea and the Philippines, Acanthacaris tenuirama is in New Caledonia, south-western New Guinea and further west (Holthuis, 1991). In spiders, Desis maxillosa (Desidae) is in New Caledonia and New Guinea. Argyrodes amboinensis (Theridiidae) is in New Caledonia, New Guinea, the Moluccas, and Sulawesi. Cyclosa albopunctata (Araneidae) is in New Caledonia, New Guinea, and Africa. In Diptera, Elephantomyia subgen. Elephantomyia (Tipulidae) is known from New Caledonia and western New Guinea. Blepharipa sugens (Tachinidae) is in New Caledonia, PNG, and Malesia. Allactoneura (Mycetophilidae) is in New Caledonia, western New Guinea, Sulawesi, Borneo and further west. In Tipulidae Limonia (Thripticomyia) subsaltens is in New Caledonia, Fiji, Samoa and PNG, and Gonomyia subgen. Gonomyia is widespread in the tropics but in Australasia/Oceania only at: New Caledonia, Fiji, eastern and western New Guinea. In beetles, the eucnemid Mesogenus austrocaledonicus of New Caledonia is sister to M. cavifrons of New Guinea (Muona, 1991) and in chrysomelids, Stethotes bertiae of New Caledonia seems closest to S. minuta of New Guinea (Jolivet et al., 2007a). Ottistira (Curculionidae) is in New Caledonia, New Guinea, and Indonesia (Kuschel, 2008). In butterflies, the New Caledonian Papilio montrouzieri and Polyura clitarchus have their closest relatives in the Papuan region (Holloway & Peters, 1976). In other Lepidoptera, Holloway (1979) cited six New Caledonian endemics that have their closest relatives in New Guinea: Epiplema lateritica (Uraniidae), Anisozyga caledonica, Chloroclystis macroaedeagus (Geometridae), Agrius fasciatus (Sphingidae), Nycteola canoides and Veia pectinata (Noctuidae). Lasioceros aroa (Notodontidae) is in New Caledonia and New Guinea, with a distinct subspecies in Fiji. Polyacme dentata (Geometridae) is on New Caledonia/ Loyalty Is., and its two closest related species are on New Guinea. Vertebrates Leatherback turtles (Dermochelys coriacea) are widespread in the world’s seas. Two animals tagged with satellite tracking devices were found to swim between New Caledonia and Morobe, PNG (Anon., 2002). 20 In birds, Vuilleumier & Gochfeld (1976) suggested that eight New Caledonian endemic species (in Accipiter, Ducula, Charmosyna, Coracina, Aplonis, Corvus, Zosterops and Erythrura) had a ‘presumed origin’ in New Guinea, reflecting putative affinities. In at least some of these the relationships are doubtful. For example, Corvus (= Physocorax) moneduloides may have no direct affinity with birds of Oceania, Australia or New Guinea (Berlioz, 1962) and, in particular, its extraordinary tool-making abilities set it apart (Weir, Kenward & Kacelnik, 2004). The moth Pareromene cheesmani (Pyralidae) of New Caledonia and northern and southern Vanuatu has its closest relatives in New Zealand (three species) and Rennell I. (P. fusca) (Gaskin, 1974). This group follows the trend of the Norfolk – New Caledonia – Rennell – Papua ridge/trench system and biogeographic connection cited in the main text. Other Rennell I. connections are shown in the following passerines. Gerygone flavolateralis (Acanthizidae) is in New Caledonia (one subspecies), the Loyalty Is. (three subspecies), northern Vanuatu (one subspecies), and Rennell I. (one subspecies) (mapped in Mayr & Diamond, 2001). Myiagra caledonica (Monarchidae) is in New Caledonia and the Loyalty Is. (two subspecies), southern Vanuatu, northern and central Vanuatu, and Rennell I. It is related to species of the southeastern Solomon Is. (San Cristobal; M. cervinicauda), Santa Cruz – Fiji (M. vanikorensis), and the Solomon Is. (M. ferrocyanea). 13. New Zealand – New Caledonia – New Guinea Notes on the main text Mearnsia (Myrt.) is sister to Carpolepis of New Caledonia, and Metrosideros s. str. of New Caledonia, New Zealand and the central Pacific islands (east and south-eastern Polynesia to Hawaii, but not in New Britain, New Guinea or the Philippines). The Pacific Metrosideros/Mearnsia clade with its internal vicariance is sister to another vicariant, Tepualia of South America/South Africa, and this whole group (tribe Metrosidereae) is in turn sister to tribe Backhousieae of eastern Australia, yet another vicariant (Wilson et al., 2005). Central and western Australia are occupied by other tribes. Wilson (1996) compared the distribution of Mearnsia with that of Xanthomyrtus (Myrt.), cited above. In Metrosideros s. str. there is clear-cut vicariance between two main groups (Wright et al., 2000), with M. cherrieri and its allies in New Caledonia, Fiji, the Solomon Is., New Ireland, and northwest in the Bonin Is., while M. excelsa and its allies occur in New Zealand, Lord Howe I., the Kermadec Is., Vanuatu, Fiji and east through Polynesia. Wright et al. noted the ‘clear geographic separation’, but rather than accepting Wilson’s (1996) vicariance explanation they regarded the non-New Caledonian species of the first group as ‘apparent vagrants’ from New Caledonia and the species of the second group as dispersed from New Zealand. Nevertheless, this does not account for the most fundamental aspect of the pattern – the striking allopatry of the two Metrosideros groups, nor does it explain the presence of Mearnsia but not Metrosideros in New Guinea and the Philippines, or the absence of both from Australia. Wilson (1996) noted that the presence of Metrosideros on the Bonin Is. is ‘remarkable’ and that ‘standard long-distance dispersal cannot account for its occurrence’ there. He also referred to similar distributions in other taxa such as the palm Clinostigma (ranging from the Bonin and Caroline Is. to Fiji). Wright et al. (2000) found that the Bonin Islands Metrosideros is closest to a Fijian species, confirming a pattern similar to that of Clinostigma. 21 Lower plants Fungi showing the track (from Horak, 1983) include Marasmius cylindraceo-campanulatus: New Zealand, New Caledonia, PNG, Java; and Cuphocybe: New Zealand, New Caledonia, New Guinea. This track also appears in the range of Rozites: New Zealand, New Caledonia, New Guinea, Chile, southern Argentina (12 species), and two species in the Northern Hemisphere; and Entoloma haastii: New Zealand, New Caledonia, PNG, Chile, southern Argentina, Europe. In lichens, Megalospora sulphureorufa of Lord Howe and New Caledonia is related to M. bartlettii of New Zealand (North Cape) and M. granulans of New Guinea (Fig. 5; Sipman, 1983). The only Lord Howe specimen is placed in the same species as the New Caledonian one, but shows similarities with the New Guinea species. Pseudocyphellaria poculifera is in northern New Zealand, Lord Howe I., Norfolk I., New Caledonia, Fiji, New Guinea (Morobe), and also Peninsular Malaysia and Uganda (Galloway, 1994). The liverwort group Acromastigum sect. Acromastigum is in New Zealand, New Caledonia, and Hawaii, while the related A. subgen. Triandrophyllopsis is endemic to Mt. Wilhelm, PNG (Schuster, 1969). The moss group Hypnodendron sect. Sciadocladus occurs in New Zealand, New Caledonia and the Solomon Islands (not in New Guinea) (Tangney, 1990). The fern ally Tmesipteris (Australia – Pacific) is represented in PNG by a single species on Bougainville I. (northern Solomon Is.), Goodenough I. (D’Entrecasteaux Is.) and Isuarava (mainland PNG north-east of Mt. Albert Edward). It appears to be related to T. sigmatifolia of New Zealand and New Caledonia (Johns & Bellamy, 1981). Seed plants Hypericum gramineum shows a ‘morphological and geographical trend’ along the line: New Zealand, New Caledonia, New Guinea, Taiwan (Robson, 1981). Lagenophora lanata (Compositae) is in New Zealand (north of Auckland), New Caledonia, New Guinea (widespread through the mountains), Malesia and India (Koster, 1966). Invertebrates Westermann (2000) described marine faunal realms of the Mesozoic (based largely on invertebrates) and for the Indo-Pacific Realm proposed as ‘an acceptable minimum range’: New Zealand, New Caledonia, New Guinea, and the Himalayas. The landsnail Papulaoma monticola (Punctidae) and its immediate relatives occur in New Zealand, New Caledonia and New Guinea (F.M. Climo, pers. comm., 20 – v – 2007). The spider Orsinome (Tetragnathidae) is in New Zealand, New Caledonia, New Guinea, and east to Madagascar. In Diptera, Thoracochaeta brachystoma (Sphaeroceridae) is in New Caledonia, New Zealand, PNG, and the Holarctic. Outer arc groups absent in New Caledonia Seed plants Examples include the palms Metroxylon, Gulubia, Calamus, Physokentia and Hydriastele s. lat., Paramapania (Cyper.), the family Dichapetalaceae, Melastomataceae tribe Astronieae, Spiraeanthemum s. str. (Cunon.) (Fig. 2; Pillon et al., 2009), Myristica (Myristica.), Eurya (Thea.), Gunnera (Gunner.; montane in the tropics), Coriaria (Coriaria.; montane in the tropics), Parasponia (Ulm.), Haplolobus (Burser.), Dracontomelon (Anacard.), Pometia (Sapind.), 22 Melicope sect. Lepta ‘dioecious group’ (Rut.) (Hartley, 2000), Atuna (Chrysobalan.), Endospermum (Euphorb.), Dillenia (Dillen.), Burckella and Palaquium (Sapot.), Weinmannia sect. Fasciculata (Cunon.), Anacolosa (Olac.), Osmoxylon (Aral.) and Dolicholobium (Rub.). Many of these are also absent in Queensland (Metroxylon, Gulubia, Calamus, Paramapania, Gunnera, Coriaria, Parasponia, Astronieae, Eurya, Haplolobus, Dracontomelon, Pometia, Atuna, Burckella, Weinmannia, Anacolosa, Osmoxylon and Dolicholobium). Gunnera and Coriaria, both on the outer arcs (and in New Zealand), are only found in montane sites in the tropics and may be absent from New Caledonia for ecological reasons. Invertebrates Solem (1958) cited ‘very great differences and only a few similarities’ between the land snails of New Caledonia and Vanuatu. For example, two high level taxa of the ‘Palaeo-Oriental fauna’ (Pupinidae, Trochomorphinae) and two of the ‘Pacific Ocean fauna’ (Partulidae, Microcystinae) reach Vanuatu but not New Caledonia. In Homoptera, Fennah (1969) observed an ‘abrupt discontinuity’ between New Caledonia and Vanuatu, with some groups present in New Caledonia, Australia etc. but not on the islands to the north-east (Vanuatu etc.). Other groups of Vanuatu, the Solomon Is., and Fiji are absent from New Caledonia. In Tipulidae (Diptera), Hynes (1993) highlighted several notable absences from New Caledonia, for example, Styringomyia didyma, otherwise widespread in the Pacific in Melanesia (New Guinea, Solomon Is., Vanuatu, Fiji), Polynesia (Samoa, Hawaii, French Polyesia) and Micronesia. It is replaced on New Caledonia by two endemic Styringomyia species. Other examples of fauna present on the outer Melanesian arc but absent from New Caledonia include the coral genus Zoopilus (Hoeksema, 1989), the cone shells Conus glaucus, C. nimbosus, C. proximus, and C. gloriamaris (Röckel et al., 1995), the Pacific landsnail family Partulidae (Caroline Is. east to the Marquesas) (Cowie, 1992), and the cicada subtribe Cosmopsaltriina (Duffels & Turner, 2002). Examples in Diptera include the genera Japenoides (Tabanidae) (Mackerras, 1971), Acropsilus (Dolichopodidae), Dissoptera (Syrphidae), Actinochaetopteryx and Cavillatrix (Tachinidae), and Zygothrica (Drosophilidae), the polymorphic species Mimegralla albimana (Micropezidae; Hennig, 1966), and in mosquitoes Uranotaenia, the Mansonia crassipes group and the Aedes (subgen. Stegomyia) scutellaris group (the last found throughout the South Pacific except New Caledonia and New Zealand) (Belkin, 1962; Taylor & Maffi, 1978). Examples in Homoptera include Lamenia and Phaciocephalus (Fennah, 1969), in beetles the elateroid Maelodorus (Muona, 1991) and the tribe Crinotarsini (Cerambycidae; Gressitt, 1961), and in spiders Conothele (Ctenizidae) and Nihoa (Barychelidae). Vertebrates Taxa include, in fishes, the blenniids Blenniella caudolineata and Istiblennius bellus (Springer & Williams, 1994), the clupeids Amblygaster sirm and Sardinella melanura (Clupeidae) (Whitehead, 1985) and the lutjanids Lutjanus boutton, L. johnii, and L. timorensis (Allen, 1985); in birds, the kingfisher Todiramphus (Halcyon) chloris: Red Sea to Indonesia, New Guinea, the Solomon Is., Vanuatu (south to Aneityum) and Fiji/Tonga/Samoa, and Aerodramus (Collocalia) vanikorensis (Apodidae), widespread from the Philippines and through Melanesia to Vanuatu (del Hoyo et al., 1999), and in bats, the globally widespread families Hipposideridae and Molossidae (Nowak, 1999). The absence in New Caledonia of genera such as Chaerophon (Molossidae) and Emballonura (Emballonuridae), both widespread in the south-west Pacific including Vanuatu and 23 Fiji, may perhaps reflect insufficient collecting (Parnaby, 2002) or they may be endemic to the outer arc track. 14. New Caledonia/outer arc vicariants Seed plants Gonystylus (Thymel.) occurs through Malesia from Sumatra to the Solomon Is. and Fiji, and its sister, Arnhemia, is endemic to the Northern Territory of Australia. The clade is absent in New Caledonia but is replaced there by its sister group, comprising Deltaria and Solmsia both endemic to New Caledonia. The sister of this whole clade is Lethedon of New Caledonia (15 species) and Queensland (Beaumont et al., 2009). Corynocarpus similis (Corynocarp.) of New Guinea, the Bismarck Archipelago, the Solomon Is. and Vanuatu vicariates neatly with a clade in New Caledonia (C. dissimilis) and New Zealand (C. laevigatus) (Wagstaff & Dawson, 2000). Arytera brackenridgei (Sapind.) of the Solomon Is., Vanuatu, and Fiji/Tonga/Samoa vicariates with three New Caledonian endemics (A. gracilipes, A. lepidota, A. arcuata) (van Balgooy et al., 1996). In Guioa (Sapind.), a clade on the Solomon Is., Vanuatu, Fiji and Tonga (G. sufusana etc.) vicariates with one on Australia, Lord Howe and New Caledonia (G. microsepala etc.) (van Balgooy et al., 1996). Morierina (Rub.), endemic to New Caledonia, is closely related morphologically to Badusa of Fiji, Tonga, Vanuatu, Solomon Is., Biak I. (off north-western New Guinea), Palau I. and the Philippines (Jansen, 1984) (Motley et al., 2005, instead had Morierina in a clade with New Caledonian endemic species of Bikkia but the only Badusa in their sample was the Palau species). 15. New Caledonia – Vanuatu Notes on the main text The two species of Tinadendron (from Achille, 2006) are: T. noumeanum: Noumea Peninsula, New Caledonia, thicket and sclerophyll forest on calcareous terrain at the edge of the sea, up to 50 m. T. kajewskii of the Loyalty Is. (Maré) and southern Vanuatu (Erromango and Aneityum), at both localities in dense humid forest on uplifted coral, up to perhaps 300 m. Examples of the Loyalty Is. – Vanuatu connection described recently include the landsnail Sturanya sublaevigatoides (Helicinidae) of the Loyalty Is. (all three islands) which is most similar to S. sublaevigata of Vanuatu (Tanna north to Banks and Torres Is.) (Richling, 2009) and the dipteran Bactrocera trilineola (Tephritidae) known only from the Loyalty Is. (Lifou, Mare) and Vanuatu (Mille, 2008). Lower plants New Caledonia/Vanuatu liverworts include: Acromastigum filum (in Vanuatu on Tanna), Bazzania bernieri (I. des Pins, Aneityum) and Lepidozia aubertii (in Vanuatu on Tanna). Schistochila integerrima is known from New Caledonia and Aneityum (So, 2003). Mosses restricted to the two areas include Campylopus verrucosus (in Vanuatu on Santo), Euptychium neo-caledonicum (Aneityum), E. dumosum, Fissidens subacutissimus, Glossadelphus obscurus (Santo), Leucobryum neo-caledonicum, Macromitrium pulchrum, Mittenothamnium nano-operculatum (Pentecost), Pogonatum circinatum (Aneityum, Santo), Pterobryella rigida (Aneityum), Rhizogonium medium 24 (I. des Pins, Vanuatu), Symphysodontella cylindracea var. attenuata, Trichosteleum neocaledonicum, and T. subrhinophyllum (Banks). Rhizogonium trachypelma is in the Kermadec Is., New Caledonia and Vanuatu (Malekula). In ferns Sphenomeris deltoidea, Trichomanes assimile, T. laetum, Humata pusilla, Thelypteris herusiana, Cyathea vieillardii, Blechnum obtusatum, and B. gibbum (all from Aubréville et al., 1967-) and Sphaerostephanos richardsii (Holttum, 1977) are all restricted to New Caledonia and Vanuatu. Orthiopteris firma is in these islands and there are also possible records from Fiji (western Viti Levu). Seed plants Heliconia indica var. austrocaledonica (Helicon.) is in southern New Caledonia, the Loyalty Is., and western Vanuatu (mapped by Kress, 1990). Orchids with similar patterns include the following examples from Aubréville et al. (1967-) and Lewis & Cribb (1989). Goodyera subregularis: New Caledonia (southern Grande Terre) and Vanuatu (throughout the east, north to the Santa Cruz Is. Megastylis: endemic to New Caledonia (7 species) and Vanuatu (one species, on Aneityum). Moerenhoutia grandiflora: New Caledonia and Vanuatu (Aneityum and Efate). Dipodium punctatum var. squamatum: New Caledonia (widespread on Grande Terre and I. des Pins) and Vanuatu (Aneityum and Erromango). Spathoglottis petri: north-eastern New Caledonia and Vanuatu (widespread). Diplocaulobium ouhinnae: New Caledonia (north-eastern Grande Terre) and Vanuatu (northern and central). Bulbophyllum neocaledonicum: New Caledonia (disjunct: southern and north-eastern Grande Terre) and Vanuatu (Santo). B. atrorubens: New Caledonia (central and north-eastern Grande Terre) and Vanuatu (Santo). Zeuxine vieillardii: New Caledonia (widespread on Grande Terre) and Vanuatu (Tanna and Santo). Phaius robertsii: New Caledonia (disjunct: south and north-eastern Grande Terre) and Vanuatu (Banks Is.: Vanua Lava). In eudicots, Ficus ser. Austrocaledonicae (Moraceae) is in New Caledonia, Loyalty Is. and Vanuatu (Tanna, Aneityum, Erromango, and Ambrym) (Corner, 1970). Arthroclianthus (Leguminosae) is in New Caledonia and Vanuatu (Lewis et al., 2005). Dizygotheca (Aral.) (= Schefflera ‘Pacific’ clade, subgroup Dizygotheca) is in New Caledonia and southern Vanuatu (Erromango, Tanna) (Plunkett et al., 2005; Lowry, 1989). Other New Caledonia/Vanuatu groups are ‘Strobilopanax’ (Aral.; = Meryta pro parte, Tronchet et al., 2005; Thorne, 1969) and Oxera (Labiatae) (de Kok & Mabberley, 1999). Polyscias schmidii (Aral.) of southern Vanuatu (Erromango) appears to be closely related to the New Caledonian P. nitida and P. nothisii (Lowry, 1989; Eibl et al., 2001). Phyllanthus myrianthus (Phyllanth.) of southern Vanuatu appears to be most closely related to New Caledonian species (Webster, 1986). Santalum austrocaledonicum (Santal.) is in New Caledonia, the Loyalty Is. and southern Vanuatu (Aneityum, Erromango). Vitex collina (Labiatae) is restricted to New Caledonia and southern Vanuatu (Aneityum, Erromango, and Efaté) (Mabberley, 1998), as are Sarcomelicope simplicifolia subsp. neo-scotica (Rut.; in Vanuatu on Aneityum and Erromango) (Hartley, 1982) and Drypetes deplanchei subsp. deplanchei (in Vanuatu on Aneityum) (Green, 1990). Dysoxylum bijugum (Mel.) is on Norfolk I., New Caledonia, and Vanuatu (Mabberley, 1998). Other New Caledonia – Vanuatu species include Trachymene cussonii (Umbellif.) (Green, 1979), Alyxia 25 podocarpa (Apocyn.; Middleton, 2002), Arytera neoebudensis (Sapind.; van Balgooy et al., 1996), and Lotus anfractuosus (Legum., Kramina & Sokoloff, 2004; in New Caledonia only on the Loyalty Is. and I. des Pins), Haloragis prostrata (Halorag.), Euphorbia pancheri (Euphorb.), and Trichospermum inmac (Tilia.) (the last three from Aubréville et al., 1967-) Invertebrates In nudibranchs, Phyllidiopsis neocaledonica of New Caledonia and P. vanuatuensis of Vanuatu are sister species, separated by a trench 6000-7000 m deep ‘that might have caused fragmentation in the original range and subsequent speciation of the two’ (Valdés, 2001a). P. holothuriana is on the Norfolk Ridge 150 km southeast of Grande Terre, and southern Vanuatu (Aneityum) (Valdés, 2001b). The mesogastropod Eatonina heliciformis is in New Caledonia and central Vanuatu (Ponder & Yoo, 1980). Solem (1958) stressed the great difference between the snail faunas of New Caledonia and Vanuatu (the ‘Pacific’ families of Mollusca that reach Vanuatu but not New Caledonia are cited above). Among the ‘few similarities’ he found was Draparnaudia singularis, only known from the two countries. In spiders, Tangaroa dissimilis (Opell, 1983), Argiope caledonia (Levi, 1983), Arctosa konei (Lycosidae) and Dolomedes titan (Pisauridae) are restricted to New Caledonia and Vanuatu (in A. konei New Caledonia has one subspecies, Vanuatu the other). In Diptera, Idiohelius and Limonia (Goniodineura) apicifusca (Tipulidae) and Brachytarsina rouxi (Streblidae) have the same distribution, and the Tripteroides caledonicus group of mosquitoes occurs in New Caledonia, the Loyalty Is., Vanuatu, Santa Cruz Is., and Rotuma (north of Fiji) (Taylor & Maffi, 1978). In Hemiptera, the marine water strider Halobates katherinae is in New Caledonia and Vanuatu (sister to a Fiji and Tonga pair of species) (Andersen, 1991), as is the genus Gnostocoris, which belongs to a Tasman Sea family of ‘obviously… ancient, primitive relicts’ (Monteith, 1979). New Caledonia – Vanuatu Diptera include Dolichopus melanesiana (Dolichopodidae): New Caledonia and Vanuatu (Bickel, 2008). Examples from beetles include the weevils Mauia subnotata (Anthribidae) (New Caledonia, Loyalty Is., Vanuatu, the genus Baladaeus (Curculionidae): New Caledonia, Loyalty Is., Vanuatu, and the genus Tetragynetes (Curculionidae) (Kuschel, 2008). Tetragynetes comprises two species, T. dispar: Loyalty Is. (Lifou, Mare), I. des Pins, Vanuatu (Eromango, Aneityum, Tanna), and T. onychialis: Vanuatu (Ambrym). Kuschel wrote that the genus ‘is only remotely related to Platysimus’, a central Pacific genus (see section 22 below) but is placed next to it in his treatment. In Hymenoptera, Orasema koghisiana (Heraty, 1995), Pseudofoenus ritae (Jennings & Austin, 2002) and Theronia simillima (Gupta, 1962) are restricted to New Caledonia and Vanuatu. In beetles, the buprestid Metataenia subgen. Mroczkowskia is in New Caledonia and Aneityum (R. Holynski, pers. comm. 18 – viii – 2005). In butterflies, Anaphaeis java peristhene (Papilionidae) is in Norfolk I., New Caledonia, the Loyalty Is., and Vanuatu, Danaus hamata moderata (Danaidae) is in New Caledonia and Vanuatu, D. pumila is in both these plus the Loyalty Is. and I. des Pins, Euploea sylvester tristis (Danaidae) is in New Caledonia and Vanuatu (plus Santa Cruz Is.), E. nemertes iphianassa has the same range, E. treitschkei jessica is in New Caledonia and Vanuatu, Luthrodes cleotas excellens and Nacaduba biocellata armillata (Lycaenidae) are in New Caledonia, Loyalty Is. and Vanuatu (Holloway & Peters, 1976). In other Lepidoptera, Ozola plana (Geometridae) of New Caledonia forms a ‘rather isolated section’ with O. achrophaea and O. hesperias of Vanuatu (Holloway, 1979). Cleora immemorata (Geometridae), Cephonodes lifuensis (Sphingidae), Agape lifuensis, 26 Asota alienata (Hypsidae), Calathusa basirufa, Parallelia cunelineata (Noctuidae) are all on New Caledonia and Vanuatu, and (with the exception of Asota alienata) the Loyalty Is. (Holloway, 1979). Pareromene cheesmani (Pyralidae) is on New Caledonia and Vanuatu (Gaskin, 1974). Vertebrates In amphidromous freshwater fishes, Lentipes kaaea (Gobiidae) is in New Caledonia and Vanuatu (www.fishbase.org) as are the bleniid reef fishes, Ecsenius tessera and E. isos (Bleniidae) (Springer, 1999); the tripterygiid Enneapterygius rhothion is in New Caledonia and southern Vanuatu (Erromango) (Fricke, 2002). In birds, Ptilinopus greyii (Columbidae) is in New Caledonia, Loyalty Is., Vanuatu, and the Santa Cruz Is. Lichmera incana (Meliphagidae) is in New Caledonia (one subspecies), Loyalty Is. (two subspecies), and Vanuatu (two subspecies). In Clytorhynchus (Fig. 10), C. pachycephaloides (Monarchidae) is in New Caledonia and Vanuatu (Erromango north to Banks Is). The other three species in the genus are C. vitiensis of Fiji, Rotuma, Tonga and Samoa, C. nigrogularis of Fiji and Santa Cruz, and C. hamlini of Rennell I. Relatives of Clytorhynchus include Neolalage, endemic to Vanuatu (Efate to Banks Is.), and Mayrornis of Fiji and Santa Cruz Is. These ranges all indicate an important break by Santa Cruz and Banks Is. (Distributions of Vanuatu birds are taken here from Bregulla, 1992, rather than Dickinson, 2003). Other birds restricted to New Caledonia and Vanuatu include Accipiter fasciatus vigilax (Accipitridae), Chalcophaps indica sandwichensis (Columbidae) (including Banks and Santa Cruz Is.), Turdus poliocephalus pritzbueri (Lifu and Tanna), Lalage leucopyga simillima (Loyalty Is. and southern Vanuatu) and Coracina caledonica (Grande Terre, Loyalty Is., Erromango, Malekula and Santo). Phylidonyris (Guadalcanaria) undulata (Meliphagidae) of New Caledonia and P. notabilis of Vanuatu (central and Santo) are closely related (Diamond & Marshall, 1976) and sometimes separated as genus Glycifohia (Dickinson, 2003); (Phylidonyris is then restricted to Australia). 16. New Caledonia – Vanuatu – Fiji Takhtajan (1986) wrote that a ‘curious connection’ exists between the floras of New Caledonia and those of Vanuatu and Fiji. This area of endemism is ‘curious’ because it would not be predicted using the model of dispersal from Asia and Australia that Takhtajan and most authors have followed. Lower plants The moss Ectropothecium pacificum and the fern Leptopteris wilkesiana are both restricted to New Caledonia, Vanuatu, and Fiji/Samoa. Seed plants The following orchids are all endemic to New Caledonia, Vanuatu and specified localities in the Fiji/Samoa/Tonga area (Lewis & Cribb, 1989): Microtatorchis schlechteri (Fiji; Kores, 1991, treated the Fiji material as a separate species), Erythrodes oxyglossa (Fiji, Wallis and Futuna Samoa, Tonga), Goodyera rubicunda var. triandra (Fiji, Samoa), Earina valida (Fiji, Samoa), Bulbophyllum samoanum (Fiji, Samoa), Pristiglottis montana (Samoa), Coelogyne lycastoides (Fiji, Samoa, Tonga; Clayton, 2002), Peristylus novoebudarum (Fiji/Niuafou), and Didymoplexa 27 micradenia (Fiji, Samoa, Tonga; Kores, 1991; Lewis & Cribb, 1989, also included a Javanese species in the synonymy). Examples from dicots include the following. Geissois s. str. (Cunon.): New Caledonia, Vanuatu (Vanua Lava to Aneityum), Santa Cruz Is., and Fiji (Pillon, 2008). Balanops sparsifolia (Balanop.): New Caledonia, keyed with B. pedicellata of Vanuatu and Fiji (Carlquist, 1980; the only other locality for the genus is in the Cairns area, north-eastern Queensland). Ficus scabra (Mor.): New Caledonia, Vanuatu, and Fiji/Samoa/Tonga (Smith, 1979-1996). Piliocalyx (Myrt.): New Caledonia, southern Vanuatu (Erromango), and Fiji (Smith, 1979-96; Craven, 2001; Craven et al., 2006). Leucopogon septentrionalis (Eric.): New Caledonia, Vanuatu, and Fiji (Smith, 1979-1996). Manilkara dissecta (Sapot.): New Caledonia, Vanuatu, and Fiji/Samoa/Tonga (Smith, 19791996). Acacia simplex (Legum.) has the same range (Smith, 1979-1996). Schefflera ‘Pacific clade’, ‘subgroup Gabriellae’ (Aral.): New Caledonia, southern Vanuatu (Aneityum), and Fiji (Plunkett et al., 2005; Lowry, 1989). Cyclophyllum (Rub.): New Caledonia, Vanuatu, and Fiji (Morat, Jaffré & Veillon, 2001). Plectranthus forsteri (Labia.): New Caledonia, Vanuatu, and Fiji/Tonga (Smith, 1979-1996). Invertebrates Insects in New Caledonia, Vanuatu and Fiji include, in Diptera Bactrocera (Bactrocera) curvipennis (Tephritidae), in Homoptera Plestia oceanica (also Samoa; Fennah, 1969), in beetles Cnemidothrex (Curculionidae (Kuschel, 2008), in butterflies Hasora khoda khoda (Hesperidae), a related group of subspecies in Appias albina (including A. a. psyche) (Papilionidae) (also Samoa/Tonga), Euploea tulliolus forsteri (southern Vanuatu) and Jamides carissima susana (Lycaenidae) (Holloway & Peters, 1976), and in moths Gymnoscelis sara and Scardamia eucampta (Geometridae), Parallelia prisca (also Samoa/Tonga), Hypena fijiensis (Noctuidae) and Xenothictus/Xeneda (Tortricidae; the two may be congeneric) (Holloway, 1979). Vertebrates Taxa endemic to New Caledonia – Vanuatu – Fiji (and often including nearby Samoa and Tonga) include the reef fishes Helcogramma hudsoni (Tripterygiidae) (also Samoa and Kiribati; Fricke, 2002), Ecsenius fourmanoiri (also Lord Howe I. and southern Tonga, in Fiji only in the Lau Group; Springer, 2002), and the bird Rhipidura spilodera (Rhipiduridae) (northern and central Vanuatu). In bats, Notopteris is a ‘very ancient, long-isolated lineage’ (Flannery, 1995) that occurs in northern New Caledonia (N. neocaledonica), southern Vanuatu and Fiji (N. macdonaldi) and Tonga (as subfossils). 17. New Caledonia – Fiji/Samoa/Tonga Lower plants In mosses, Trichostomum insulare is in New Caledonia and Fiji (Viti Levu), Leucobryum tenuifolium and Distichophyllum vitianum are in New Caledonia, Fiji and Samoa. In liverworts, Lophocolea parva, Megaceros monospirus, Schusterella parhami and Telaranea bisetula are in New Caledonia and Fiji, Lopholejeunea renistipula is known only from I. des Pins and Fiji. The ferns Ctenopteris crassifrons and Dictymia mckeei are both in New Caledonia and Fiji, as are 28 Lindsaea moorei (in Fiji only on Mt. Korobaba) and Belvisia melanesica (in Fiji only on Mt. Tomanivi). Asplenium carruthersii of Fiji is very close to A. oligolepidum of New Caledonia (Brownlie, 1977). Christella pacifica ranges in New Caledonia, Fiji, and Samoa (Holttum, 1977), Schizaea melanesica is in New Caledonia, Fiji, and Tonga (Brownlie, 1977). Seed plants Hibbertia lucens (Dillen.) is restricted to New Caledonia and Fiji. Smith (1978, 1979-1996) regarded this distribution as ‘very unusual’ in plant species. He wrote that ‘Because of the presumably independent origin of the two archipelagoes in the remote past, it seems likely that in this case one or more disseminules travelled from New Caledonia to Fiji comparatively recently (in terms of geological time) and that there has not yet been time for independent evolutionary directions to have become apparent’. In fact, the geological history of New Caledonia and Fiji may not have been as independent as Smith suggested (see main text). Thorne (1969) cited Buraeavia (Picrodendr.) (currently placed in the more widespread Austrobuxus) as a New Caledonia – Fiji group but Smith (1979-1996) and Airy Shaw (1980) regarded the Fijian species as closer to the New Guinea species than to any in New Caledonia (cf. section 14, above). Acmopyle (Podocarp.) is extant in New Caledonia and Fiji (there are fossil records from Australia, Antarctica and Chile; Hill & Brodribb, 1999, also New Zealand; Pole, 2007). The orchids Anoectochilus imitans, Octarrhena oberonioides, Phreatia pachyphylla, and Nervilia platychila are all restricted to New Caledonia and Fiji (Kores, 1991). Hetaeria whitmeei and Bulbophyllum pachyanthum are on New Caledonia and Fiji, Samoa and Tonga (Kores, 1991). Planchonella sphaerocarpa had some weak support as sister of P. membranacea of Fiji (Swenson et al., 2007). Polyscias joskei (Aral.) of Fiji is sister to a group of New Caledonian species (Eibl et al., 2001). Diospyros pancheri (Eben.) of New Caledonia was regarded as closest to D. foliosa of Fiji by Kostermans (1977). Passiflora barclayi (Passiflor.) is only known from New Caledonia and Fiji. (De Wilde, 1972, synonymised the species under the more widespread P. aurantia, while Green, 1972, Smith, 1979-1996, and Jaffré et al., 2001, recognised P. barclayi as distinct). The genus Pytinicarpa (Compositae) is restricted to New Caledonia (the species were treated under Brachycome in Jaffré et al., 2001) and Fiji (treated under Keysseria in Smith, 1979-1996) (Nesom, 2001). In a broad sample of Pacific Planchonella species (Sapot.), P. sphaerocarpa of New Caledonia was placed with P. membranacea of Fiji (Swenson, Munzinger & Bartish, 2007; New Guinea species were not widely sampled and some might be involved here). In other examples, Podocarpus colliculatus (Podocarp.) of the I. des Pins is closest to P. pallidus of Tonga (de Laubenfels, 2005). Typical Nicotiana fragrans (Solan.) occurs on New Caledonia, the Loyalty Is. and Tonga (there is a distinct form endemic to the Marquesas). Invertebrates The spider crab Guinotinia comprises one species in New Caledonia, the other in Fiji and Tonga (Richer de Forges & Ng, 2009). In craneflies (Tipulidae), Limonia fijiana is endemic to New Caledonia, Fiji and Samoa, L. ochricapilla to New Caledonia and Fiji, in dolichopodid flies; Cymatopus baravikai of Fiji is ‘most similar in appearance’ to C. neocaledonicus of New Caledonia (Evenhuis, 2005b). In beetles, the staphylinid Megarthrus has two sister species in New Caledonia and Fiji, respectively (Cuccodoro, 1998). 29 In fulgoroid Homoptera, Suva albonotata is is New Caledonia, Fiji, Samoa and Sri Lanka (Fennah, 1969). In Lepidoptera, Anisodes lautokensis (Geometridae) is restricted to New Caledonia and Fiji, Poecilasthena leucodrya (Geometridae) is on these two and also Samoa (Holloway, 1979). Nola lichenosa (Nolidae) is in New Caledonia, Fiji and Rotuma; specimens from Vanuatu have a similar facies but may differ. Polyura gamma (Nymphalidae) of New Caledonia is related to a Fiji species (Holloway & Peters, 1976). Vertebrates Drepanoptila (Columbidae) is a monotypic genus endemic to New Caledonia (and ‘without doubt the most beautiful bird of the country’ – Delacour, 1966). Goodwin (1983) regarded it as ‘very similar’ to the orange doves of Fiji (Ptilinopus victor superspecies). Gymnomyza (Meliphagidae) occurs in New Caledonia (‘Leptomyza’), Fiji and Samoa, with one endemic species in each. 18. New Caledonia – Samoa Lower plants Taxa endemic to New Caledonia – Samoa include liverworts cited by Miller et al. (1983): Bazzania falcifolia, Cololejeunea arrectifolia, Frullania angustistipa, Heteroscyphus jackii, and Lophocolea explanata, and So (2000) adds Plagiochila bialata. Other examples include the moss Syrrhopodon polytrichoides and the fern Humata brackenridgei. Seed plants The tree Crossostylis (Rhizophor.) has one clade in New Caledonia, Samoa and French Polynesia, and a vicariant in Fiji, northern Vanuatu and the Solomon Is. (Setoguchi et al., 1998). Invertebrates Examples include the spiders Tamasesia (Mysmenidae) and Argyrodes samoensis (Theridiidae), the dolichopodid fly Helixocerus (Bickel, 2002), and the cone shell Conus exiguus (Röckel et al., 1995). In related patterns, the spider Argyrodes gracilis is in Lord Howe Is., New Caledonia, and Samoa. Thalassomya maritima (Chironimidae) is in New Caledonia and Samoa, and disjunct in Micronesia (Marshall Is, Palau, Guam, northern Mariana Is.) and Hong Kong. These connections probably run south of Fiji, as in the Crossostylis clade cited above. Nevertheless, a northern track may be shown by the moth Nola samoana (Nolidae) of New Caledonia, Vanuatu, Rotuma (north of Fiji) and Samoa. It is replaced in Fiji by N. fijiensis (Holloway, 1979). 19. New Caledonia – outer Melanesian Arc (Bismarck Archipelago, Solomon Islands, Vanuatu, Fiji/Samoa/Tonga) Notes on the main text As indicated in sections 13 and 14, many groups range widely through the outer Melanesian arc, including Vanuatu and Fiji, but avoid New Caledonia, while other Melanesian groups do occur in New Caledonia. Morat et al. (1984) cited 42 genera of New Caledonian rainforest plants that are not endemic there but do not occur in Australia, and most of these are Malesian groups. For example, Dolichandrone spathacea (Bignon.) is a back-mangrove tree dispersed in seawater that ranges almost continuously from India through Malesia to the Admiralty Is. (pers. obs.), the 30 Bismarck Archipelago, the Solomon Is. and New Caledonia (van Steenis, 1963, 1977). Van Steenis regarded it as ‘most peculiar’ and ‘most remarkable’ that the species has never been found in Australia. Two other species in a different subgenus do occur there and this pattern, with vicariants in Australia and Melanesia, is common. Kroenke’s (1996) model could also explain groups such as the ‘very primitive’ oligochaete Acanthodrilus, comprising 19 species endemic to New Caledonia and one endemic to the Kermadec Is. (Jamieson, 1981). The cone shells Conus bruuni and C. plinthis have the same New Caledonia – Kermadec Is. range (Röckel et al., 1995) and the gastropod Cerithium abditum is in New Caledonia, the Kermadec Is. and disjunct in western Malesia (Houbrick, 1992). Polyscias sect. Tieghemopanax is sister to the Australian P. elegans. Eibl et al. (2001) concluded ‘it seems likely that the common ancestor of Tieghemopanax arrived in New Caledonia [from Australia] through a single long-distance dispersal event after the island’s separation from Australia. However, because we have no reliable estimate for the age of these lineages we cannot rule out a more ancient vicariance event’. In fact the evidence for trans-oceanic dispersal can be interpreted in different ways and dispersal may only be required within Queensland, and then only if there is overlap there between Tieghemopanax and P. elegans). Polyscias cissodendron of sect. Tieghemopanax occurs on Lord Howe, New Caledonia, Vanuatu, and the Solomon Is. Eibl et al. (2001) suggested that the ‘relatively young’ age of these islands (apart from New Caledonia) coupled with the species’ being sister to, or nested in, the New Caledonian P. dioica, ‘suggests a relatively recent series of dispersal’. But the data cited do not provide evidence for this. The age of the current islands is less relevant than the age of the relevant subduction zones and the phylogenetic position of the species is not in itself unequivocal evidence for trans-oceanic dispersal, only for range expansion and overlap within New Caledonia. Eibl et al. (2001) also suggested that the sister relationship between other New Caledonian species of Polyscias and Fijian species ‘can be explained only by dispersal’, but again there is no unambiguous evidence supporting dispersal and vicariance seems more probable for this standard pattern (cf. section 17 above, Springer, 1999, 2002, on reef fishes, and Zug, 1991, on lizards). Finally, the close relationship between Polyscias species endemic to New Caledonia and Vanuatu (cf. section 15 above) is cited as evidence suggesting a third independent dispersal from New Caledonia (Plunkett et al., 2001), but again, this sister relationship and a young age of the current Vanuatu islands are just as consistent with ancient vicariance. In the sylvioid birds (Fig. 8), Mayr (1933) described the new genus Cichlornis and compared it with the New Caledonian Megalurulus (where it is currently included). On the basis of several characters Mayr distinguished the pair from Ortygocichla of New Britain and Trichocichla of Fiji. On the other hand, he concluded that the last two were synonymous, although ‘The distribution of such a genus (New Britain and Viti Levu) is certainly very paradoxical’. Mayr, 1933: 4). Subsequent authors have lumped all four genera, but the New Britain – Fiji affinity suggested by Mayr may be real and this track is shown in Fig. 8, ‘jammed’ between the ranges of Megalurulus and Cettia. Lower plants In lichens, Pseudocyphellaria desfontainii is in Africa, Sri Lanka, Malesia, and via the outer arc and New Caledonia to French Polynesia. P. prolificans is in Sri Lanka and Malesia to the Solomon Is., New Caledonia and Polynesia, plus the Kermadec Is. (Galloway, 1994). Examples from mosses (Tixier, 1979) include Camptochaete porotrichoides: New Caledonia, Vanuatu, and New Guinea, Spiridens reinwardtii: New Caledonia, Vanuatu, Samoa, Solomon Is., D’Entrecasteaux 31 Archipelago, New Guinea, and Malesia, and Macromitrium salakanum: New Caledonia, southern Vanuatu (Aneityum), New Guinea, the Philippines, and Java. In liverworts, Radula amentulosa and R. formosa both range: New Caledonia, Vanuatu, Fiji and east to the Society Is., and to the north-west in New Guinea, Malesia, and Sri Lanka (Schuster, 1984). Examples in ferns include Sphaerostephanos doodioides (Thelypterid): New Caledonia, Vanuatu, and the Solomon Is. (Holttum, 1977), and Cyathea lunulata: New Caledonia, Vanuatu, Fiji, Samoa, the Solomon Is., Bismarck Archipelago, and Caroline Is. (Holttum, 1964). Seed plants Widespread outer arc taxa not on New Caledonia were cited above. In seed plants, Thorne (1969) recorded 15 genera endemic to the outer arc and New Caledonia, and New Caledonian groups with affinities in the Solomon Is., Vanuatu, and Fiji/Samoa/Tonga (sometimes including New Zealand) were cited by Lowry (1998). Examples of taxa on the Melanesian arc including New Caledonia, but not in Australia or New Zealand, include the following. In conifers, Florin (1963) illustrated two ‘migration routes’ running between New Zealand and New Guinea, namely an inner arc: South Island – New Caledonia – Milne Bay/New Guinea mainland (section 13); and an outer arc: North Island – Vanuatu – Solomon Is. – Morobe. The third track in the region is: eastern Australia – southern New Guinea. The second, outer arc track is occupied by extant members of Dacrycarpus and Dacrydium (Podocarp.). Dacrycarpus (nine species) ranges in New Zealand, New Caledonia, Vanuatu, Fiji, Bismarck Archipelago, and through Malesia to Burma. Fossils are also known in India, Australia and South America (de Laubenfels, 1984; Hill & Brodribb, 1999). Dacrydium (21 species) is in New Zealand, New Caledonia, Fiji, Solomon Is., Bismarck Archipelago, and through Malesia. Outside this area fossils are known from southern Australia (Hill & Brodribb, 1999). The total absence of these abundant, diverse groups in modern Australia, India and South America may reflect an initial low diversity (or at least propensity for evolution) in Gondwana and a mainly Pacific history (cf. the analysis of living and fossil distribution of Nothofagus in Heads, 2006). The distribution of the pan-Melanesian groups in New Guinea is complex, reflecting the geology there; the island comprises some 32 accreted terranes. Disjunctions among New Guinea taxa on the accreted terranes are frequent and conform to standard patterns (Heads, 2001, 2003). The disjunctions and isolated records in New Guinea are often involved with broader Melanesian disjunctions. The following widespread Melanesian plants are all restricted in the New Guinea region to particular areas composed of accreted terranes (listed in italics): Bikkia pancheri (Rub.): New Britain, the Solomon Is., Vanuatu, and New Caledonia (I. des Pins) (Motley et al., 2005). Alphandia (Euphorb.): northern New Guinea (near Jayapura), New Caledonia and Vanuatu (Webster & Airy Shaw, 1971; Radcliffe-Smith, 2001). Delarbrea paradoxa (Aral.) ranges: northern PNG (Madang) (and disjunct at Timor – Aru Is.), New Britain, Solomon Is., Vanuatu, and New Caledonia (van Balgooy & Lowry, 1993). Ryssopterys timoriensis var. discolor (Malpigh.): PNG (Huon and Papuan Peninsulas), Solomon Is., Vanuatu, and New Caledonia (van Balgooy, 1966c). Cypholophus decipiens (Urtic.): northern and eastern New Guinea, New Britain, eastern Caroline Is., the Solomon Is., northern Vanuatu, and northern New Caledonia (Wilmot-Dear & Friis, 1998). Geniostoma rupestre var. rupestre Group A: New Guinea (Vogelkop Peninsula and Biak I.), Solomon Is., Vanuatu, New Caledonia, Fiji, Samoa, and Rarotonga. It also occurs further north and 32 west in the Mariana Is., Sulawesi, the Philippines and Taiwan, but it is not in Australia (Conn, 1980). Amyema artensis (Loranth.) has an interesting distribution which illustrates the break-down of the Melanesian distribution pattern into parallel arcs (cf. Figs. 6 and 7). It is in New Guinea, the Milne Bay islands, New Caledonia (the last two localities sharing a distinct form), and southernmost Vanuatu. It also occurs along an outer, disjunct arc north-east of here, on the Caroline Islands, northernmost Vanuatu, and Samoa. The conspicuous gap between the two metapopulations is filled by A. novaebrittaniae (Barlow, 1992). Other New Caledonia – Melanesian taxa absent from Australia include the following. The Cycas rumphii group (Cycad.): Malesia, throughout Melanesia (New Guinea, Bismarck Archipelago, Solomon Is., Vanuatu, New Caledonia) and Fiji/Samoa/Tonga; also extending west of Malesia to Madagascar and adjacent east Africa (Hill, 1994). Its absence from Australia despite a wide Indo-Pacific distribution is remarkable. Hugonia sect. Durandea (Lin.): New Guinea, Bismarck Archipelago, Solomon Is., northern Vanuatu, New Caledonia, and Fiji (van Balgooy, 1993b). Neuburgia (Logania.): Malesia, New Guinea, Solomon Is., New Caledonia, southern Vanuatu, and Fiji (Leenhouts, 1966). Amylotheca acuminatifolia (Loranth.): alpine western New Guinea (Wissel Lakes and Lake Habbema in the Snow Mts.), keyed with species of New Caledonia (A. pyramidata) and Vanuatu (A. banksiana) (Barlow, 1974). Heliconia indica (Helicon.): Moluccas, New Guinea, Solomon Is., Vanuatu, New Caledonia, and Fiji/Samoa (plants from Fiji and Samoa are sometimes treated separately as H. paka, e.g. by Smith, 1979-1996). Joinvillea (Flagellar.): Solomon Is., southern Vanuatu, New Caledonia, and Fiji/Samoa. The genus is also disjunct in western Malesia (not New Guinea), the Caroline Is., and Hawaii (van Balgooy, 1966b). Barringtonia Group IV (Lecythid.): Malesia, New Guinea, Bismarck Archipelago, Solomon Is., Vanuatu, New Caledonia, and Fiji (Payens, 1967). Agatea (Viol.): New Guinea, Bougainville I. (northern Solomon Is.), New Caledonia, and Fiji/Tonga (Fig. 13; Jacobs & Moore, 1971). Geniostoma rupestre var. glaberrimum (Logan.): Mariana Is., Caroline Is., Solomon Is., Vanuatu, New Caledonia, Fiji and east to Rapa I. and the Marquesas (Conn, 1980). Sarcolobus retusus (Asclep.): Sulawesi, New Guinea, Solomon Is., Caroline Is., and New Caledonia (Rintz, 1980). Rintz wrote ‘probably in N. Australia’, and ‘possibly in NE Australia’ but no evidence was given (cf. Council of the Heads of Australasian Herbaria, 2009) and absence from here would be a standard pattern. Invertebrates Corals on the outer arc include Polyphyllia novaehiberniae of New Caledonia, Vanuatu, Fiji/Samoa/Tonga, the Solomon Is., the Bismarck Archipelago and northern New Guinea (Hoeksema, 1989). Gastropods on the outer arc and New Caledonia but not in Australia include Cerithium lifuense (Houbrick, 1992), the coneshells Conus lienardi, C. cinereus, C. ochroleucus, and C. corallinus (Röckel et al., 1995), and the cowry Erronea succincta (Lorenz & Hubert, 1993). In Hemiptera the mirid genus Rubrocuneocoris is in New Caledonia, Vanuatu, Fiji/Samoa/Tonga, the Bismarck Archipelago, possibly the Solomon Is. and New Guinea, and also extends to Indo-China (Schuh, 1984). 33 In Diptera, the diverse Chrysosoma noumeanum Group (Dolichopodidae) is endemic to New Caledonia and is closely related to the C. arrogans Group of the Solomon Is., Bismarck Archipelago, and northern New Guinea (Bickel, 1994). Other diptera endemic to the arc include Dixina (Dixidae): New Caledonia and the Solomon Is., Cyclopodia oxycephala (Nycteribiidae): New Caledonia, Vanuatu, Solomon Is., and Bactrocera umbrosa (Tephritidae): New Caledonia, Vanuatu, Solomon Is., Bismarck Archipelago, and PNG, extending to the eastern Oriental Region. Tipulidae on the arc include Limonia novocaledonica: New Caledonia and the Bismarck Archipelago. Typical Limonia notata is in New Caledonia, Vanuatu, Palau, Sumatra, and the Oriental Region, while the other subspecies, L. n. ssp. Solomonis, is in New Caledonia (not Vanuatu), Solomon Is., Bismarck Archipelago, and Palau. In Tabanidae, Chasmia ranges: New Caledonia, Vanuatu, Solomon Is., eastern and western New Guinea, and Dasybasis rubricallosa of New Caledonia is related to D. mellicallosa of Santa Cruz Is., Solomon Is., and Manus I. (Mackerras, 1970). In mosquitoes, Belkin (1962) recorded an unnamed species of Culex (Lophoceraomyia) known only from I. Art in northern New Caledonia (Is. Bélep), which is ‘of considerable interest’ as it is related to species of Vanuatu and the Solomon Is. Examples in butterflies include Euploea boisduvali (Danaidae): New Caledonia, Vanuatu, Fiji, and the Solomon Is., E. treitschkei: New Caledonia, Vanuatu, Solomon Is., Bismarck Archipelago, Milne Bay islands and New Guinea, E. nemertes: New Caledonia, Vanuatu, Fiji, Solomon Is., Bismarck Archipelago and New Guinea, Hypolimnas octocula (Nymphalidae): New Caledonia, Vanuatu, Fiji, Solomon Is., Palau and the Caroline Is., Cyrestis telamon (Nymphalidae): New Caledonia, Solomon Is., Bismarck Archipelago, New Guinea and Sulawesi, Psychonotis schaeffera (Lycaenidae): New Caledonia, Solomon Is., Bismarck Archipelago, New Guinea and Malesia, and Luthrodes cleotas (Lycaenidae): New Caledonia, Vanuatu, Solomon Is., Bismarck Archipelago, New Guinea and Timor (all from Holloway & Peters, 1976). Parantica is in New Caledonia, Vanuatu, Solomon Is., Bismarck Archipelago, New Guinea and west to India (Ackery & VaneWright, 1984). Examples in moths (from Holloway, 1979) include Plecoptera violacea (Noctuidae), following the same track as far as the Moluccas, Utetheisa salomonis (Arctiidae): New Caledonia, Vanuatu, Solomon Is. and the Bismarck Archipelago, Cleora phryganeoides (Geometridae): New Caledonia, Vanuatu, Fiji, New Guinea and the Moluccas, Harita nodyana (Noctuidae): New Caledonia, Vanuatu, Fiji, Solomon Is., Bismarck Archipelago, and New Guinea, Chrysodeixis illuminata (Noctuidae): New Caledonia, Fiji/Tonga, Cook Is., Solomon Is., Bismarck Archipelago, and New Guinea, and Nigramma polionota (Noctuidae): New Caledonia, Fiji/Samoa, northern Vanuatu (Santo), Solomon Is., Milne Bay islands (Woodlark) and Manus I. The wasp Pseudofoenus ritae of New Caledonia/Vanuatu is sister to P. karimuiensis of PNG (Jennings & Austin, 2002). The termite Nasutitermes novarumhebridarum is in New Caledonia, Vanuatu, Solomon Is. and New Guinea (Roisin & Pasteels, 1996). Vertebrates The New Caledonia/outer arc fauna includes the freshwater fish family Rhyacichthyidae, comprising Protogobius: New Caledonia, and Rhyacichthys: New Caledonia, Solomon Is., Malesia and Taiwan. (Terateleotris from Laos may also belong here). The group is basal to a large, worldwide clade, the Gobioidei (Thacker & Hardman, 2005). In reef fishes, the tripterygiid Enneapterygius williamsii is in the Solomon Is., Vanuatu, New Caledonia, and Tonga (Fricke, 2002). The snappers Etelis radiosus, Lutjanus semicinctus, and Paracaeso kusakarii are along the same arc, including New Caledonia, but are not in Australia (Allen, 1985). 34 In birds, Columba vitiensis (Columbidae) is in New Guinea/Philippines, Solomon Is., Vanuatu, New Caledonia, Lord Howe, and Fiji/Samoa. The New Caledonian pigeon Ducula goliath is a member of the brenchleyi species group also in the Solomon Is., Vanuatu and Fiji (Barré et al., 2003). Coracina caledonica (Campephagidae) is in the Solomon Is., Vanuatu, and New Caledonia (mapped in Mayr & Diamond, 2001). Charmosyna (Loriidae) is in New Guinea/Moluccas, Bismarck Archipelago, Solomon Is., Vanuatu, New Caledonia (presumed extinct) and Fiji. Porphyrio porphyrio samoensis (Rallidae) is distributed throughout the Bismarck Archipelago, Solomon Is., Vanuatu, New Caledonia, and Fiji/Samoa (Ripley, 1977). Accipiter haplochrous of New Caledonia is related to A. rufitorques of Fiji, A. albogularis of the Solomon Is. (including the Santa Cruz Is.), and A. melanochlamys throughout montane New Guinea (Mayr & Diamond, 2001). (The group is absent from Vanuatu, again possibly implying a New Caledonia – Fiji – Santa Cruz dog-leg involving overlap of two tracks in Fiji). Until Mayr discerned this affinity, A. rufitorques was treated as conspecific with A. fasciatus of Australia, New Guinea, New Caledonia etc. Wattel (1973) continued to stress the relationship because ‘The Melanesian islands were undoubtedly colonized from Australia’ but this may not be correct. Two birds are distributed widely through Melanesia and on Norfolk I. (Turdus also has an extinct subspecies on Lord Howe), but are not in Australia: Lalage leucopyga (Campephagidae) of southeastern Solomon Is., Vanuatu, New Caledonia (including the Loyalty Is.) and Norfolk I., and Turdus poliocephalus (Turdidae) of Malesia, New Guinea, Solomon Is., Vanuatu, New Caledonia, Norfolk, and Fiji/Samoa. In bats, Miniopterus macrocneme is in the Bismarck Archipelago, Solomon Is., Vanuatu, and New Caledonia. 20. New Caledonia – Melanesia – Micronesia Lower plants Examples in mosses include Hyophila beruensis: Micronesia (Caroline Is., Mariana Is., Kiribati), New Caledonia, and Polynesia (Fiji/Tonga, and Tahiti), Macromitrium papillosum: Micronesia (Caroline Is., Mariana Is., Marshall Is.) and New Caledonia, and Spiridens balfourianus: Caroline Is. (Ponape, Kusaie), New Caledonia, Fiji, Rapa and the Society Is. In liverworts, Lophocolea defectistipula is in New Caledonia and the Caroline Is. (Kusaie), Lejeunea vesicata is in New Caledonia, the Marshall Is. and Borneo. Seed plants Geniostoma rupestre var. crassifolium (Logan.), endemic to New Caledonia, is closest to G. rupestre var. tongense of Fiji, Tonga, Niue and the Mariana Is. (Conn, 1980). Invertebrates Examples in cone shells include Conus luteus: New Caledonia, Tuamotu Is., Marshall Is., and the Philippines, C. polongimarumai: New Caledonia and the Marshall Is., and C. cervus: New Caledonia, the Marshall Is., and Sulawesi (Röckel et al., 1995). In spiders, Cryptothele marchei (Cryptothelidae) is in New Caledonia and the Mariana Is. In Diptera, the New Caledonian species Bactrocera caledoniensis and B. psidii form a clade with B. ochrosia of the northern Mariana Is. (Michaux & White, 1999). Rutylapa (Keroplatidae) is in New Caledonia, Micronesia, and west to Africa. Limonia pectinunguis (Tipulidae) is in New 35 Caledonia (Loyalty Is.), Palau, and the Mariana Is., and Limonia trukensis is in New Caledonia and Micronesia, as is Drosophila pallidifrons. Drosophila sulfurigaster ssp. bilimbata is in New Caledonia, Fiji/Samoa, disjunct to Micronesia (Guam, northern Mariana Is., Wake I.), and the Hawaiian Is. In marine water striders (Hemiptera), the mariannarum subgroup of the Halobates princeps group has one species in each of New Caledonia and Vanuatu, Fiji, Tonga, and Samoa, and one disjunct in the Caroline Is./southern Mariana Is. (Andersen, 1991). Vertebrates Examples from reef fishes include Microlabrichthys pascalus (Serranidae): New Caledonia east to south-eastern Polynesia and disjunct in the north-west at Micronesia, the Ryukyu Is. and southern Japan (Springer, 1982), the grouper Epinephelus retouti: New Caledonia – south-eastern Polynesia, disjunct at Palau/Japan, Sumbawa and the south-west Indian Ocean (Randall & Heemstra, 1991), and Awaous guamensis (Gobiidae): Mariana Is. and Hawaii in the north Pacific, and New Caledonia, Vanuatu and Fiji in the south. The honeyeater Myzomela cardinalis occurs on the Loyalty Is. (not Grande Terre), Vanuatu, Rotuma I., Samoa, southern Solomon Is., and in Micronesia (Palau, Mariana Is., and Caroline Is. including Truk). The Micronesian populations were ‘tentatively’ separated as M. rubatra by Dickinson (2003). 21. New Caledonia – Philippines/western Malesia Lower plants Examples include the mosses Dicnemoloma piliferum: New Caledonia, Java, Warburgiella cupressinoides: New Caledonia, Philippines (Luzon, Mindanao) (Tixier, 1979), Symphysodon subneckeroides: New Caledonia, Philippines, Trematodon papuanum: New Caledonia, Philippines, Java, and Wilsoniella decipiens: New Caledonia, Philippines, Java, Sri Lanka, and India. The same disjunction is seen in the following liverworts known from New Caledonia and the specified localities in western Malesia: Bazzania subtilis: Philippines, Frullania philippensis: Luzon, Harpalejeunea parisii: Luzon, Panay, Caudalejeunea circinata: Philippines to China, Cololejeunea gynopthalma: from the Philippines to Thailand and Sri Lanka, C. haskarliana: from the Philippines to the Mascarene Is., C. inflata: to China, Japan and Sri Lanka, C. minuta: also Sri Lanka, Japan, Dendroceros formosana: also Vietnam and China, Lopholejeunea muensis: Taiwan, and L. zollingeri: New Caledonia, Sumatra, Java and Sulawesi. An extreme variant of this track is seen in Paraschistochila gaudichaudii: New Caledonia and Penang (Peninsular Malaysia). The fern Paesia rugosula is in New Caledonia, Tahiti and the Philippines (Luzon). Invertebrates In gorgonians, Rumphella aggregata is in New Caledonia and the Moluccas, while Pterostenella comprises one species of New Caledonia and one in the Philippines and Réunion I. (Grasshoff & Bargibant, 2001). The gastropod Microvoluta joloensis: New Caledonia/Tonga – Philippines – Madagascar, was cited above. The spider Rogmocrypta (Salticidae) ranges: New Caledonia, Philippines, and Singapore. Examples in Diptera include Omapanta quadrimaculata (Phoridae): New Caledonia, Sulawesi, Taiwan, Episyrphus viridaureus (Syrphidae): New Caledonia, Java, and the Malay Peninsula, and Drosophila atriplex (Drosophilidae): New Caledonia, Philippines, and the Oriental Region. In 36 spiders, Telemofila (Telemidae) is known from New Caledonia and Sumatra, and Gasteracantha lunata (Araneidae) is in New Caledonia, Moluccas, and Timor. G. rubrospinis has a similar range: New Caledonia, the Moluccas, Guam, Lombok, and Sulawesi. Similar disjunction between New Caledonia and Philippines (often including Taiwan etc.) is seen in 12 cone shells: Conus sanzaka, C. capitanellus, C. ione, C. profundorum, C. darkini, C. dusaveli, C. kuroharai, C. chiangi, C. dayriti, C. kimioi, C. aphrodite, and C. pagodus (the last is also in the Red Sea) (Röckel et al., 1995). In other gastropods, Cerithium abditum occurs around New Caledonia/ Kermadec Is., and disjunct at the Philippines/Sulawesi/Borneo, C. ophioderma is at New Caledonia/ Kermadec Is. and disjunct at the Philippines/southern Japan (Houbrick, 1992) and the cowry Nesiocypraea teramachii is at New Caledonia and the Philippines/southern Japan (Lorenz & Hubert, 1993). The lobster Nephropsis acanthura is found off New Caledonia, northeastern Queensland, the Philippines and Madagascar (Holthuis, 1991). The spider Dictyna (Dictynidae) is in Africa, the Palearctic, India, China, Burma, Philippines, New Caledonia, New Zealand, Hawaii/North America, South America (not New Guinea, Australia or Polynesia). The coral Cantharellus noumeae is in New Caledonia (Hoeksema, 1989), north-eastern Queensland and Madang, PNG (GBIF), and (fossil) in Borneo (Hoeksema, 1989). The only other member of the genus is recorded from the Red Sea (Hoeksema, 1989). Vertebrates This pattern is seen in reef fishes such as Myripristis melanostictus: New Caledonia, Philippines/Moluccas, and the serranid Microlabrichthys lori: New Caledonia east to the French Polynesia, also Philippines/Palau (Springer, 1982). In Tripterygiidae, Fricke (2002) cited species with south-west Pacific (New Caledonia/Vanuatu) – north-west Pacific (Ryukyu Is., Taiwan/Philippines) disjunctions; examples include Enneapterygius rubicauda: Loyalty Is., Vanuatu, Taiwan, Philippines, Ryukyu Is. Likewise, the emperor fish Lethrinus ‘sp. 2’ is known only from the Loyalty Is. and the Philippines (Carpenter & Allen, 1989). 22. New Caledonia – central South Pacific Notes on the main text New Caledonia has been seen as a centre of origin for many other groups; for example, Holloway (1979) wrote that the tortricine moths Xenothictis/Xeneda ‘appear to have radiated in New Caledonia and spread to other areas of the Pacific’, and in Cupaniopsis (cited in the main text), Adema (1991) proposed New Caledonia as a centre of origin for Pacific species. In Meryta (Aral.), Tronchet et al. (2005) suggested that because the New Caledonian M. denhami lies phylogenetically among other volcanic island taxa, it is derived by recent back-migration from a centre of origin held by these. Instead, the diversity of Meryta in New Caledonia may represent a series of accretions of arcs and floras onto the continental crust. Although one member of the central Pacific clade (M. denhami) has already been accreted onto New Caledonia, the other members survive on and around central Pacific arcs and volcanic centres (Micronesia – Vanuatu – eastern Polynesia) and remain unaccreted, at least for now. Lower plants Examples in mosses include Racopilum spectabile: Malesia, Micronesia (Caroline Is., Mariana Is.), Solomon Is., Vanuatu, New Caledonia, Fiji/Samoa with the typical subspecies, and in New Caledonia and Tahiti, each with endemic subspecies, Rhizogonium setosum: New Guinea, 37 Vanuatu, New Caledonia, Fiji/Samoa, and the Society Is., Macromitrium tongense: New Guinea, New Caledonia (I. des Pins), Lord Howe, Fiji/Tonga and Henderson I. (near Pitcairn), Orthorrhynchium cylindricum: Loyalty Is., Vanuatu, Fiji/Tonga/Samoa, Australs, Society Is., and Marquesas, Papillaria helictophylla: New Caledonia, Vanuatu, Wallis & Futuna, the Society Is., and the Marquesas, Syrrhopodon mammillatus: New Caledonia, Fiji/Samoa, and the Society Is. (Raiatea), S. banksii: New Caledonia, Fiji and Samoa to Micronesia and the Marquesas (Fisher, 2006), Vesicularia aperta: New Caledonia, Vanuatu, Wallis & Futuna, and Tahiti (with a queried record from Hawaii). Thuidium obtusifolium var. neocaledonicum is endemic to New Caledonia, the type variety is in southern Vanuatu (Erromango), Austral, Society, Tuamotu, Gambier (incl. Pitcairn and Henderson) and Marquesas Is. Examples in liverworts include Dendroceros tahitiensis: New Caledonia and the Society Is. (Tahiti and Moorea), Cheilolejeunea cookiensis: Louisiade Archipelago (south-eastern New Guinea), New Caledonia, Fiji/Samoa, Cook Is. (Rarotonga) and Society Is. (Tahiti) and Porella viridissima: Norfolk I. and New Caledonia east to Tahiti (So, 2002). In pteridophytes, Tmesipteris lanceolata of New Zealand and New Caledonia is related to T. norfolkensis of Norfolk I. and T. gracilis of the Society Is. and Marquesas (Chinnock, 2003). Examples in ferns (records from Aubréville et al., 1967-) include Culcita straminea: New Caledonia, Vanuatu, Fiji and central Polynesia, Antrophyum alatum: New Caledonia, Fiji/Samoa and Tahiti, Teratophyllum wilkesianum: New Caledonia, Samoa and Tahiti, Ctenopteris blechnoides: New Caledonia to Polynesia, Paesia rugosula: Philippines (Luzon), New Caledonia and Tahiti (it resembles P. scaberula of New Zealand), Grammitis neocaledonica of New Caledonia, which is very close to G. hookeri of ‘Polynesia’, and Cyathea alata of New Caledonia, which is related to C. decurrens of Vanuatu, Fiji/Samoa, the Cook Is. and Society Is. (Holttum, 1964). Seed plants In Trimenia (Trimen.), the tree species range from Sulawesi through New Guinea, the Solomon Is., and Fiji to the Marquesas. As mentioned, the single New Caledonian species is a tree. It may belong to a Coral Sea track as its pollen shows affinities with the liane species, found in northeastern New South Wales (the MMO) and disjunct in PNG (Philipson, 1986). Monocot examples of New Caledonia – central Pacific endemism include the orchid genera Earina: New Zealand (in the three main islands plus Chatham Is.; two species), New Caledonia (three species), and one species each in Vanuatu, Fiji, Samoa and Tahiti, and Microtatorchis: New Caledonia, New Guinea, Philippines, the Caroline Is., Fiji, and Samoa to the Society Is. (Smith, 1979-1996). Other monocots with a New Caledonia – central Pacific range include Cenchrus calyculatus (Gram.): New Caledonia, Micronesia, east to the Marquesas, Oberonia equitans (Orchid.): New Caledonia and Vanuatu east to the Tuamotu Is., and Taeniophyllum fasciola (Orchid.): New Caledonia east to the Society Is. Other examples include the following eudicots. Ficus prolixa (Mor.): Micronesia, Vanuatu and New Caledonia (not the Solomon Is.), east to south-eastern Polynesia (Smith, 1979-1996). Schleinitzia (= Prosopis insularum, Mimos.): Philippines, New Guinea, Solomon Is., Vanuatu, New Caledonia, and Fiji – south-eastern Polynesia (de Vogel, 1975; Lewis et al., 2005). Homalanthus nutans (Euphorb.): Caroline Is., New Caledonia, Vanuatu and Society Is. 38 Pisonia sect. Paucistaminatae (Nyctagin.): New Guinea (4 species), and one species in each of the Solomon Is., New Caledonia, Society Is. and Hawaii (Stemmerik, 1964). Capparis nummularia (Capparid.): Mariana Is., Palau and other Caroline Is., Mussau I. north of New Ireland (but not New Ireland, New Britain, or New Guinea), Solomon Is., New Caledonia, and Fiji to the Tuamotu Is. and Pitcairn I. (St. John, 1965). Canavalia sericea (Legum.): from the Caroline Is. to Fiji/Samoa/Tonga and east to the Society Is., with southwestern outliers at New Caledonia (I. des Pins) and Queensland (Sauer, 1964). Weinmannia sect. Leiospermum: New Zealand, New Caledonia, Vanuatu, Bismarck Archipelago (and Sulawesi), east to the Marquesas (Hopkins & Bradford, 1998). Crossostylis (Rhizophor.): New Caledonia, northern Vanuatu, Solomon Is., and Fiji east to the Marquesas. Morat et al. (1984) suggested Crossostylis and Earina had a ‘Pacific island origin’. Both genera show notable allopatry among their respective species and also with related genera and so a vicariance origin of the pattern is likely. Grewia crenata (Tilia.): New Caledonia, the Loyalty Is., Vanuatu, Fiji, and east to the Society Is. Morinda myrtifolia (Rub.): New Caledonia (four varieties) east to the Tuamotu Is. and Marquesas (Smith, 1979-1996). Hedyotis foetida (Rub.): New Caledonia to the Austral Is. (Smith, 1979-1996). Nicotiana fragrans (Solan.) of New Caledonia (southern Grande Terre, Loyalty Is.), Tonga and the Marquesas. In Alstonia (Apocyn.) a large clade comprises 12 New Caledonian endemics, plus A. costata of New Caledonia, Vanuatu and the Solomon Is., east to the Marquesas (Sidiyasa, 1998). This central Pacific group is sister to a species of eastern Australia. Invertebrates In freshwater shrimps a New Caledonia – south-eastern Polynesia clade was recovered in the genus Caridina (Page et al., 2007). These authors cited ‘surprisingly good dispersal abilities’ to explain patterns in this genus but, again, chance processes cannot explain the central Pacific endemism and its standard boundary at the old plate margin: another clade of Caridina is in Okinawa, Malesia, and Australia, vicariant to the west of the central Pacific group. In spiders, the genus Tangaroa (Uloboridae) comprises one species in the Caroline Is. (Yap), one in New Caledonia/Vanuatu, and a third in south-eastern Polynesia (Tahiti, Rapa) (Opell, 1983). A central Pacific clade of Dicaea (Thomisidae) in New Caledonia, New Zealand, Fiji, Tonga and the Austral Is. (including Rapa) vicariates neatly with Mimusenops: Society Is., Marquesas, Hawaii, and the Americas (Garb & Gillespie, 2006). The authors suggested that the pattern is due to islandhopping across the Pacific, but this does not account for the very precise east Pacific/west Pacific split around a node in south-eastern Polynesia. Examples in Diptera include the Bactrocera atra-passiflorae complex: New Caledonia, Tuvalu, and Fiji east to French Polynesia (Michaux & White, 1999), Abbemyia baylaci (Dolichopodidae): New Caledonia, Vanuatu, east to French Polynesia (Bickel, 2002), vicariating with the other two congeners in eastern Australia, and Limonia subgenus Doaneomyia (Tipulidae): New Caledonia, Vanuatu, Fiji, and the Society Is. Examples from beetles (all Curculionidae, from Kuschel, 2008) include Platysimus: Solomon Is. and Kiribati to New Caledonia, Kermadec Is., Austral and Society Is., Viticis: southern Ryukyu Is. (Ishigaki I. and Iriomote I., off Taiwan) (this species keys out first suggesting it may be a separate clade), New Caledonia, Loyalty Is., Vanuatu (Santo to Aneityum), Fiji, and one species in the Cook Is. (Mangaia) and Marquesas (Nuku Hiva), and Elytroteinus geophilus: north-eastern New 39 Caledonia (Balade), Vanuatu (Epi), Fiji (Lakemba, Moce only), Wallis, Samoa (Upolu), Tonga (Tongatapu, Vavau), and Cook Is. (Rarotonga, Aitutaki). The species is close to E. vitiensis: Fiji (Nandarivatu, 1000m). Examples in butterflies include Badamia atrox (Hesperidae): New Caledonia, Vanuatu, Fiji and Marquesas, Euploea lewinii: New Caledonia, Vanuatu and the Santa Cruz Is., east to the Society Is., and Hypolimnas antilope lutescens (Nymphalidae): New Caledonia, Vanuatu, Fiji/Samoa/Tonga and the Cook Is. (all from Holloway & Peters, 1976). In other Lepidoptera, Anisodes samoana (Geometridae) is in New Caledonia, the Loyalty Is., Vanuatu, Fiji/Samoa/Tonga, and the Society Is. (Holloway, 1979). Vertebrates In amphidromous freshwater fishes, the genus Lentipes (Gobiidae) is in Africa, Indonesia, and the Pacific, where it occupies the Ryukyu Is. and Hawaii in the north, and New Guinea, New Caledonia, Vanuatu and the Marquesas in the south, each with endemic species (www.fishbase.org). The petrel Pseudobulweria rostrata has breeding records from New Caledonia and the Solomon Is. east to the Marquesas. Other Central Pacific endemic birds include the parrot Cyanoramphus (New Zealand – New Caledonia – Society Is.) (see main text and notes, section 11). 23. New Caledonia – Polynesia – Hawaii Lower plants Liverwort examples include Acromastigum sect. Acromastigum: New Zealand, New Caledonia, and Hawaii, Cololejeunea ceatocarpa: Réunion, New Caledonia and Hawaii, Lophocolea autoica: New Caledonia and Hawaii, and Bazzania inaequabilis: New Caledonia, Samoa, and Hawaii (with a queried record in the Philippines). The moss Holomitrium seticalycinum is only recorded in New Caledonia and Hawaii. Seed plants In Freycinetia sect. Freycinetia (Pandan.) Stone (1973) recognised a group of species from New Zealand and Norfolk I. (F. baueriana), New Caledonia (F. longispica), Rarotonga (F. wilderi), Rapa I. (F. rapensis), and Hawaii (F. arborea). This central Pacific affinity is distributed very differently from two other Freycinetia clades in New Caledonia, sect. Solmsiella of New Caledonia and New Guinea (cf. secton 11) and sect. Warburgiella: New Guinea, Solomon Is., Vanuatu, and New Caledonia (section 19) (Stone, 1981). In a sample of spiny solanums (Solanum sect. Leptostemonum), the New Caledonian S. pancheri is sister to two Hawaiian species in a strongly supported clade that is sister to and vicariant with Australian species (Levin et al., 2006). The Hawaiian species of Melicope sect. Pelea (Rut.) are closest to the New Caledonian M. vieillardii (Hartley, 2000); this clade’s relatives occur in south-eastern Polynesia. (New Caledonian species were not sampled in the molecular study of Harbaugh et al., 2009.) In a sample of Pacific Pittosporum species (Pittospor.), New Caledonian species formed a clade with species of Fiji, Tonga and Hawaii (Gemmill et al., 2002). 40 Lysimachia mauritiana (Primul.) occurs in the central Pacific only on New Caledonia, Rapa I., and Hawaii. It is also in China, Japan and the Mariana Is., and disjunct in Mauritius and Réunion in the south-west Indian Ocean (van Balgooy & Bentvelzen, 1984). In an example of character, rather than taxon, geography, leaf heteroblasty – pronounced changes between juvenile and adult leaves – is especially common in plants of Hawaii, New Caledonia, New Zealand and the Mascarenes (Burns & Dawson, 2006). The authors also observed that the heteroblasty in New Zealand and New Caledonia species is similar despite the different climates, ‘and the phenomenon likely results from some aspect of their shared geological history’. Invertebrates Examples in molluscs include the cone shell Conus smirna: New Caledonia, Kermadec Is., and Hawaii (Röckel, 1995) and the cowry Blasicrura rashleighana, with one subspecies on New Caledonia and the only other one on Hawaii (Lorenz & Hubert, 1993). The hypogeal shrimp Ligur uveae is known from the Loyalty Is., Fiji, Tuvalu, Hawaii, Moluccas/Philippines and the Aldabra Is. north of Madagascar (Maciolek, 1983). In the cosmopolitan beetle genus Rhantus (Dytiscidae), the ‘pacificus group’ is definitely known from New Zealand, New Caledonia, the Solomon Is., New Guinea, and Hawaii (Balke et al., 2007b; certain species from Chile/Argentina, Assam, and West Africa may perhaps also belong here, M. Balke, in litt. 8 – ii – 2007). Vertebrates In the morwongs cited above, Cheilodactylus francisi of Lord Howe, New Caledonia and the Kermadec Is. is most closely related to C. vittatus of Hawaii, and these two are related to an Easter I. species. The monotypic snapper genus Randallichthys is known from New Caledonia, Hawaii and Okinawa (Allen, 1985). 24. New Caledonia (/Melanesia) – South America Some documentation for a New Caledonia – tropical South America track was given in a previous paper (Heads, 2006). Lower plants In fungi, Amparoina spinosissima is recorded from New Caledonia, Argentina, Colombia (Horak, 1983) and Japan (as Mycena spinosissima; Takahashi, 1998) giving records in the four corners of the Pacific, but not in Australia. In liverworts, Dendroceros brasiliensis is in New Caledonia, Brazil, and the West Indies (Cuba, St Vincent, Guadeloupe) and Frullania bicornuta in New Zealand (North Island), New Caledonia, Juan Fernandez, Chile, Patagonia and Fuegia. In mosses, Himantocladium bauerlenii is in New Guinea, New Caledonia, North and South America. In ferns, Schizaea intermedia of New Caledonia and S. wagneri of New Guinea/Malesia are related to S. germani and relatives in tropical America (Selling, 1946). In this group the Old World and New World taxa show parallel clines, with the largest spores in both occurring in the south. In the tree fern Cyathea, the ‘Cyathea clade’ (Large & Braggins, 2004) is in New Guinea, eastern Australia, New Caledonia east to Frrench Polynesia, and in tropical South America. 41 Seed plants The podocarp Retrophyllum Page (= Podocarpus sect. Polypodiopsis, = Decussocarpus de Laub. sect. Decussocarpus ) (extant and fossil) has a very similar Melanesia – South America pattern, with extant species in New Caledonia, Fiji, New Guinea, Moluccas, and tropical South America (Peru to Colombia and Venezuela), and fossil species in Australia, New Zealand and Chile (Herbert et al., 2002). In Hernandia subgen. Hernandia (Hernand.; Kubitzki, 1970) the moerenhoutiana Group ranges: New Caledonia, Vanuatu, Solomon Is., Fiji, east to the Society Is., and also in Nicaragua, Jamaica and Hispaniola. This Pacific group is vicariant with the bivalvis Group, endemic to the McPherson-Macleay Overlap. The orchid Erythrodes ranges in north-eastern India, China, south-east Asia, Malesia, New Guinea, Vanuatu, New Caledonia, and Fiji/Tonga/Samoa, and is most closely related to Microchilus, widespread in the Galapagos and tropical America (Ormerod & Cribb, 2003b). Astelia subgenus Asteliopsis (Astelia.) comprises sect. Isoneuron in northern New Zealand/New Caledonia, sect. Periastelia in Hawaii/Marquesas/Rapa (these two sections form group A), and sect. Micrastelia of Patagonia/Falkland Is. (Skottsberg, 1937). (The mainly South Pacific genus Astelia is abundant in many forests as well as alpine communities. A detailed revision is long overdue and would be of great interest). Heliconia (Helicon.) occurs in the south-west Pacific (1-2 species; New Caledonia, Vanuatu, Solomon Is., New Guinea, Moluccas, and Fiji, Samoa) and in tropical America (50-200 species; Mexico, West Indies to southern Brazil) (Kress, 1990). Citronella (Icacin.) is in Malesia, eastern Australia, New Caledonia, Fiji/Samoa/Tonga, central Chile (30-38ºS), Brazil, north to Ecuador (Guayaquil), and disjunct in Panama/Costa Rica (de Vogel, 1975). In eudicots, the family Oncothecaceae, monotypic in New Caledonia, and the Metteniusaceae of Costa Rica to Ecuador, mainly in Colombia, form the first and second basal branches (a transPacific sequence) in a cosmopolitan clade of Lamiales, Solanales etc. or alternatively are sisters (a trans-Pacific clade) (González & Rudall, 2007, González et al., 2007). The legumes include at least two examples (Lewis et al., 2005). Castanospermum of northeastern Australia, New Caledonia, and Vanuatu is sister to Alexa of Venezuela, the Guianas, and Amazonian Brazil. Schleinitzia, known from the Philippines to New Caledonia and Society Is. (including the Mariana Is. but not Australia), is in a well-supported clade with Kanaloa of Hawaii (Kaho’olawe) and Desmanthus, in warm America and the Caribbean. In Proteaceae, Sleumerodendron of New Caledonia is sister to Euplassa with 20 species of tropical South America (Willis, 2007; cf. Virot in Aubréville et al., 1967-). Kermadecia of New Caledonia and Turrillia of Vanuatu and Fiji may also belong here (cf. Weston & Barker, 2006) but were not sampled by Willis; their exact position does not affect the trans-tropical Pacific disjunct track in Sleumerodendron/Euplassa and they may even belong to it. In the diverse, pantropical genus Diospyros (Eben.), ‘clade Q’ of Duangjai et al. (2006; equivalent to clade XI of Duangjai et al., 2009) has a trans-tropical Pacific distribution in southeast Asia, New Caledonia, and tropical America including the Caribbean. Lagenophora (Compos.) is recorded from India to New Zealand, New Caledonia, Hawaii, Juan Fernandez Is./southern Chile/Falkland Is., then disjunct at southern Guatemala, Panama and northwestern Venezuela (near Tachira) (van Balgooy, 1975). Nothofagus subgen. Brassospora is extant only in New Caledonia – New Guinea, where it is abundant and diverse, and the only member of Nothofaus present. There is also fossil material of Brassospora in New Zealand, Australia and Antarctica, but this shows less diversity than the 42 extant group (Heads, 2006). Its sister group is subgen. Nothofagus, extant on South America (the main massing of all species is South America) and with fossils in Australia. Stereocaryum (Myrt.) is in New Caledonia and the Malay Peninsula (Scott, 1980) and represents an ‘odd’, ‘unexplained’ and ‘phytogeographically enigmatic’ distribution (Johnson & Briggs, 1984). The genus is related to the very large, mainly South American Eugenia, although it ‘can hardly be a late derivative of Eugenia itself’ (Johnson & Briggs, 1984). Stereocaryum has also been compared with the South American – West Indian Calycorectes (Briggs & Johnson, 1979). Craven (2001) suggested that Stereocaryum belongs in Eugenia s. str., which is centred in Central and South America but also has rich representation in New Caledonia (36 species) and Sri Lanka (22 species), with lesser concentrations elsewhere in the Old World. Rhizophora mangle (Rhizophor.) is in New Caledonia, Fiji/Samoa/Tonga, Galapagos, Central America (west coast) and West Africa (Ding Hou & van Steenis, 1963). Halophila decipiens var. pubescens (Hydrocharit.) is in New Caledonia, Tahiti and the Caribbean (and Sri Lanka). It is replaced by the typical variety in Australia, Malesia and Asia (den Hartog, 1966). Invertebrates In millipedes, Jeekel (1986) recognised that Agathodesmus of New South Wales, Queensland and New Caledonia (Mesibov, 2009) includes a species from Jamaica. Some authors had suggested human transport to New Caledonia and Jamaica, but the New Caledonian species is endemic (Mesibov, 2009) and Hoffmann (1999) reported a second Jamaican species in the genus. Hoffmann wrote ‘while a multiple transport of rare and localised species from the Antipodes to a single West Indian island is not impossible, it does appear improbable’. The New Caledonia – Jamaica connection is instead related here to the Pacific history of the Caribbean plate and the large igneous plateaus in the central Pacific. In spiders, Aucana (Pholcidae) is in New Caledonia and central Chile (Antofagasta to Bío-Bío) (Huber, 2000). In Trichoptera, the tribe Grumichellini comprises Triplexa of southeastern Australia, Gracilipsodes of New Caledonia, and Atanatolica, Grumichella and Amazonatolica of tropical America: north-westernmost Argentina (Jujuy) and southeastern Brazil north to Costa Rica, and in the Lesser Antilles (Dominica) (Holzenthal & Oliveira Pes, 2004; Malm & Johanson, 2008). The scale insect Diaspis casuarinae is endemic to New Caledonia and is an ‘unusual’ species; it ‘bears some relationship’ with D. chilensis of Chile (Williams & Watson, 1988; www.sel.barc.usda.gov/scalenet/scalenet.htm). In Lepidoptera, Holloway (1993) cited a possible South American connection between the New Caledonian sphingid Compsulyx and the neotropical Adhemarius. In chrysomelid beetles Zira of New Caledonia is closer to Lioplacis and other Brazilian genera than to any Australia/New Zealand taxa (P. Jolivet, pers. comm. 17-xi-2006). Vertebrates The Meiolaniidae are extinct turtles up to 3m long with cranial horns and frills and a club tail. These bizarre, fossil, marine animals show a trans-tropical Pacific distribution similar to that of the extant, terrestrial plants cited above. Meiolania is known from the Miocene and Pleistocene of the Northern Territory, eastern Australia, Lord Howe I. (where species survived until 2000 years ago), and New Caledonia, including the Loyalty Is. and Walpole I. (south-east off Grande Terre). The rest of the family comprises two other genera restricted to Queensland and one genus from the Eocene of Argentina (Gaffney, 1996). The Walpole I. specimens seem to belong to a locally 43 endemic form and Gaffney suggested the family represented a relatively archaic Pacific biota that survived on young oceanic islands. He suggested that this took place by the process of biogeographic ‘hopskotch’ (McKenna, 1983), in which populations on volcanic islands survive in situ by colonising nearby new islands as the older ones erode and subside. The process is probably fundamental for Pacific biogeography. In snakes, the subfamily Boinae occurs in Madagascar (2 genera), Mauritius (2 genera), Melanesia (Candoia, formerly Enygrus) and tropical America (4 genera). Candoia ranges in Sulawesi, Palau, New Guinea, Bismarck Archipelago, Solomon Is., Vanuatu, New Caledonia (Loyalty Is. only) and Fiji/Samoa/Tonga. Burbrink (2005) found Candoia to be the sister group of the Madagascar genera and this whole clade sister to the American genera, giving either transAtlantic connections, trans-Pacific connections, or both. A trans-tropical Pacific link would involve the usual New Caledonia/Fiji – central America/Antilles disjunction. In any case, Burbrink (2005) regarded the group as relictual and the biogeographic pattern derived from distributions established prior to Gondwana breakup. The skink Emoia cyanura also has a trans-tropical Pacific range, occurring on the Loyalty Is. (not Grande Terre), Vanuatu, Solomon Is. and Bismarck Archipelago east to Polynesia and Peru (Bauer & Sadlier, 2000). Three birds, each usually treated in its own family, are often regarded as related: Rhynochetos of New Caledonia, its sister Aptornis (fossil) of New Zealand, and the sister of these two, the sunbittern Eurypyga of tropical America (Guatemala – Brazil). Livezey (1998) found a sister relationship between a clade comprising Rhynochetos and Aptornis and one comprising Eurypyga and Messelornis of the Eocene of the north Atlantic (Wyoming and Germany), indicating both trans-Pacific and trans-Atlantic links. ANALYSIS OF NEW CALEDONIAN ONISCIDEA (TERRESTRIAL ISOPODS) The following data were extracted from the global check-list of terrestrial isopods (Schmalfuss, 2009). These illustrate how much biogeographic information can be obtained from studies based on extensive global collecting and traditional taxonomic methods, even without detailed phylogeny or molecular analyses. There are nine genera endemic to New Caledonia (Caledonillo, Emydodillo, Heroldia, Mesodillo, Nesoniscus, Ochetodillo, Oroscia, Pseudosphaerillo, Wahrbergia). Other genera present in New Caledonia show interesting distributions outside the country, as follows (numbers of species in brackets). Australiodillo: Lord Howe I. (5), Queensland, New South Wales (1), New Caledonia (2). Track 5. Merulana: Eastern Australia (Cape York to Victoria) (7), New Caledonia (2), Chatham Is. (2). Track 6. Merulanella: Burma (1), Flores I. (Indonesia) (3), New Caledonia and Loyalty Is. (3). Track 21. Nagurus sundaicus: China; Indonesia: Sumatra, Java, Sulawesi; Loyalty Is.; Hawaii. Tracks 21 and 23. Neodillo: New Guinea (1), New Caledonia (1). Track 12. Nesodillo: Sri Lanka (1), south-western India (1), north-eastern India, Burma, Taiwan, Ryukyu Is. (the single named species in these countries probably comprises several species), Borneo (1), 44 Taiwan (1), Gilbert Is. (1), Burma (2), Flores (1), Aru Is. (1), New Guinea (2), New Caledonia and Loyalty Is. (8). Cf. track 21. Orodillo: New Caledonia (1), Taiwan (1). Track 21. Plymophiloscia: eastern Australia (Queensland; Melbourne) (3), Tasmania (4), New Caledonia (1). Track 6. Pseudolaureola: St Helena I. (1), Madagascar (1), Western Australia (1), New Caledonia (1). Track 1. Pyrgoniscus: Kenya (1), Tanzania (1), Madagascar (2), Lord Howe I. (2), New Caledonia (1). Track 1. Schismadillo: New Guinea (2), Queensland (1), Victoria (1), New Caledonia (1). Track 6. Scyphax: Victoria (1), New Zealand (1), New Caledonia (1). Track 5. Spherillo pygmaeus: New Caledonia, Marquesas Is. (there are other central Pacific species of Spherillo). Track 22. Xestodillo: Vanuatu and Loyalty Is. (1), New Caledonia and Loyalty Is. (1), New Caledonia (1). Track 15. CORRELATED LOCAL AND GLOBAL PATTERNS OF NEW CALEDONIAN GROUPS Loyalty Is. – central Pacific This pattern is exemplified by Cyrtandra (Fig. 9, main text). Another clear example is in the passerine Myzomela (Meliphagidae), with M. sanguinolenta on Grande Terre, Australia – Sulawesi, and its close relative M. cardinalis on the Loyalty Is., Melanesia and Micronesia (but not mainland New Guinea). The differentiation between the Loyalty Is. and Grande Terre is discussed elsewhere (Heads, 2008). Two examples have been retrieved since that paper was written. The dragonfly Xiphiagron cyanomelas (Coenagrionidae) is known from ‘the Paramalayan islands to the west of Sumatra’ to the Solomon Islands and the Loyalty Islands, but not on Grande Terre (where the genus is absent) (Lieftinck, 1976). The hornbills are occur throughout the Old World tropics to the Solomon Islands and are also known fossil on the Loyalty Islands, but there are no records on Grande Terre (Steadman, 2006). Steadman suggested that ‘it must have lived also on Grande Terre’ (p. 159) but absence of Loyalty Islands groups from Grande Terre is a common pattern. North-eastern Grande Terre – Vanuatu/Fiji The fern Lindsaea francii of north-eastern Grande Terre is distinct from all its New Caledonian congeners but very close to Vanuatu species. A similar pattern occurs in four orchids (Phreatia pachyphylla, Octarrhena oberonioides, Nervilia platychila, Anoectochilus imitans) that are present but very rare in Fiji and are each recorded elsewhere only in New Caledonia (Kores in Smith, 1979-1996), where they are all present in north-eastern Grande Terre. Another orchid, Diplocaulobium ou-hinnae, is endemic to north-eastern Grande Terre and closest to D. tipuliferum of Fiji. Orchids known in New Caledonia only from north-eastern Grande Terre and also in Vanuatu are Spathoglottis petri and Thrixspermum adenotrichum (cited as ‘Thrixspermum sp.’ in 45 the Flora; also in New Guinea and the Solomon Is.). Trichospermum inmac (Tilia.) is in northeastern Grande Terre and Vanuatu. In other plants Bulbinella pachyanthum (Lilia.), disjunct between north-eastern and southern Grande Terre, is also in Fiji/Samoa/Tonga, Erythrodes oxyglossa (Orchid.), disjunct between north-eastern and southern Grande Terre, is also in Vanuatu and Fiji/Samoa/Tonga. In insects, the noctuid moth Hypena fijiensis is known from Mt. Panié in north-eastern Grande Terre, the Loyalty Is., Vanuatu and Fiji (Holloway, 1979). The freshwater fish Rhyacichthys is in north-eastern Grande Terre (R. guilberti, cited above), the Solomon Is., Malesia, and Taiwan (R. aspro). North-western Grande Terre – localities to the west The only Pacific Island member of the rice genus Oryza is O. neocaledonica, endemic to northwestern Grande Terre (Pouembout). Its closest relative is probably O. meyeriana of Malesia (Morat et al., 1994). Albizia guillainii (Mimos.), found along the west coast, is not related to Asian or Australian congeners, but to African ones (sect. Zygia). Southern Grande Terre – New Zealand Affinities with New Zealand are seen in the freshwater fish Galaxias neocaledonicus and the tree Beilschmiedia neocaledonica (Laur.), all endemic to southernmost Grande Terre and all related to New Zealand species (G. paucispondylus, B. tarairi and Libocedrus spp.). The tree Strasburgeria (Strasburger.) is endemic to the southern Grande Terre ultramafics (Oginuma et al., 2006) and is closest to Ixerba (the only other member of the family) of northern New Zealand. Korthalsella salicornioides (Visc.) is in New Zealand, Madagascar, and southern New Caledonia (I. des Pins and near Kouaoua on the south-eastern Grande Terre coast; Aubréville et al., 1967–present). Southern Grande Terre and New Guinea Bulbophyllum lophoglottis (Orchid.) of the southern massif is very closely related to B. plumula of New Guinea, and similarly the rare liverwort Herbertus leratii, endemic to Mt. Mou in the southern massif, is probably closest to a New Guinea/Indonesian species (So, 2003). A parallel is also seen in lichens, with Megalospora hillii of southern Grande Terre closest to M. weberi of Mt Wilhelm in the PNG Highlands (Fig. 5; Sipman, 1983). REFERENCES Achille, F. (2006) Tinadendron, nouveau genre de Rubiaceae, Guattardeae de Mélanésie orientale. Adansonia, series 3, 28, 167-180. Ackery, P.R. & Vane-Wright, R.I. (1984) Milkweed butterflies: their cladistics and biology. British Museum (Natural History), London. Adema, F.A.C.B. (1991) Cupaniopsis Rdlk. (Sapindaceae): a monograph. Leiden Botanical Series, 15, 1-190. Airy Shaw, H.K. (1980) Euphorbiaceae of New Guinea. Royal Botanic Gardens, Kew, UK. 46 Allen, G.R. (1985) Snappers of the world. FAO Species Catalogue, 6, 1-208. Allen, G.R, Steene, R. & Allen, M. (1998) A guide to angelfishes and butterflyfishes. Odyssey/Tropical Reef Resesearch, Singapore. Andersen, N.M. (1991) Cladistic biogeography of marine water striders (Insecta, Hemiptera) in the Indo-Pacific. Australian Systematic Botany, 4, 151-163. Andersen, N.M. & Polhemus, D.A. (2003) A new genus of terrestrial Mesoveliidae from the Seychelles (Hemiptera: Gerromorpha). Journal of the New York Entomological Society, 111, 12-21. Anonymous (2002) Leatherbacks traced to New Caledonia. Post-Courier [Port Moresby] July 4 2002. 125, 13. Arbogast, B.S., Drovetski, S.V., Curry, R.L., Boag, P.T., Suetin, G., Grant, P.R., Grant, B.R. & Anderson, D.J. (2006) The origin and diversification of Galapagos mockingbirds. Evolution, 60, 370-382. Aubréville, A., Leroy, J.-F., Morat, Ph. & MacKee, H.S. (eds) (1967–present) Flore de la Nouvelle-Calédonie et dépendances. Muséum National d’Histoire Naturelle, Paris. Avé, W. (1984) Alyxia. Pacific Plant Areas, 4, 142-143. Avé, W. & Balgooy, M.M.J. van (1984) Euroschinus. Pacific Plant Areas, 4, 134-135. van Balgooy, M.M.J. (1966a) Dracophyllum. Pacific Plant Areas, 2, 80-81. van Balgooy, M.M.J. (1966b) Joinvillea. Pacific Plant Areas, 2, 106-107. van Balgooy, M.M.J. (1966c) Ryssopterys. Pacific Plant Areas, 2, 200-201. van Balgooy, M.M.J. (1971) Plant geography of the Pacific. Blumea Supplement, 6, 1-222. van Balgooy, M.M.J. (1975) Lagenophora. Pacific Plant Areas, 3, 150-151. van Balgooy, M.M.J. (1984) Coronanthereae. Pacific Plant Areas, 4, 186-187. van Balgooy, M.M.J. (1993a) Arthrophyllum. Pacific Plant Areas, 5, 92-93. van Balgooy, M.M.J. (1993b) Hugonia. Pacific Plant Areas, 5, 132-133. van Balgooy, M.M.J. (1993c) Hedycarya. Pacific Plant Areas, 5, 148-149. van Balgooy, M.M.J. (1993d) Tapeinosperma. Pacific Plant Areas, 5, 150-151. van Balgooy, M.M.J, Bentvelzen, P.A.J. (1984) Lysimachia mauritiana, L. decurrens. Pacific Plant Areas, 2, 154-157. van Balgooy, M.M.J. & Franken, N.A.P. (1984) Austrobuxus. Pacific Plant Areas, 4, 178-179. van Balgooy, M.M.J. & Lowry, P.P., II. (1993) Delarbrea. Pacific Plant Areas, 5, 94-97. van Balgooy, M.M.J. & Nielsen, I. (1993) Archidendropsis. Pacific Plant Areas, 5, 124-125. van Balgooy, M.M.J., Hovenkamp, P.H. & van Welzen, P.C. (1996) Phytogeography of the Pacific – floristic and historical distribution patterns in plants. The origin and evolution of Pacific Island biotas, New Guinea to Polynesia: patterns and processes (ed. by A. Keast and S.E. Miller), pp. 191-213. SPB Academic, Amsterdam. Balke, M., Pons, J., Ribera, I., Sagata, K. & Vogler, A.P. (2007a) Infrequent and unidirectional colonization of hyperdiverse Papuadytes diving beetles in New Caledonia and New Guinea. Molecular Phylogenetics and Evolution, 42, 505-516. Balke, M., Wewalka, G., Alarie, Y. & Ribera, I. (2007b) Molecular phylogeny of Pacific Island Colymbetinae: radiation of New Caledonian and Fijian species (Coleoptera, Dytiscidae) Zoologica Scripta, 36, 173–200. Barlow, B.A. (1974) A revision of the Loranthaceae of New Guinea and the south-western Pcific. Australian Journal of Botany, 22, 531-621. Barlow, B.A. (1992) Conspectus of the genus Amyema Tieghem (Loranthaceae). Blumea, 36, 293381. 47 Barré, N., de Garine Wichatitsky, M., Lecoq, R. & Maillard, J.-C. (2003) Contribution to the knowledge of the New Caledonian imperial pigeon Ducula goliath (Gray 1859) with emphasis on sexual dimorphism. Notornis, 50, 155-160. Bartish, I.V., Swenson, U., Munzinger, J. & Anderberg, A. (2005) Phylogenetic relationships among New Caledonian Sapotaceae (Ericales): molecular evidence for generic polyphyly and repeated dispersal. American Journal of Botany, 92, 667-673. Bauer, A.M. & Sadlier, R.A. (2000) The herpetofauna of New Caledonia. Society for the Study of Amphibians and Reptiles, Ithaca, New York. Beaumont, A.J., Edwards, T.J., Manning, J., Maurin, O., Rautenbach, M., Motsi, M.C., Fay, M.F., Chase, M.W. & van der Bank, M. (2009) Gnidia (Thymelaeaceae) is not monophyletic: taxonomic implications for Thymelaeoideae and a partial new generic taxonomy for Gnidia. Botanical Journal of the Linnean Society, 160, 402–417. Begg, J.G. & Grant-Mackie, J.A. (2003) New Zealand and New Caledonian Triassic Pleurotomariidae. Journal of the Royal Society of New Zealand, 33, 223-268. Belkin, J.N. (1962) The mosquitoes of the South Pacific (Diptera, Culicidae). University of California Press, Berkeley, CA. Berlioz, J. (1962) Les caractères de la faune avienne en Nouvelle-Calédonie. Comptes Rendus de la Société de Biogéographie, 39, 65-69. Bickel, D.J. (1994) The Australian Sciapodinae (Diptera: Dolichopodidae), with a review of the Oriental and Australasian faunas, and a world conspectus of the subfamily. Records of the Australian Museum Supplement, 21, 1-394. Bickel, D.J. (1999) Australian Antyx Meuffels and Grootaert and the New Caledonian connection (Diptera: Dolichopodidae). Australian Journal of Entomology, 38, 168-175. Bickel, D.J. (2002) The Sciapodinae of New Caledonia (Diptera: Dolichopodidae). Zoologia Neocaledonica Vol. 5. Systématique et endémisme en Nouvelle-Calédonie (ed. by T. Najt and P. Grandcolas). Mémoires du Muséum National d’Histoire Naturelle, 187, 11-83. Bickel, D.J. (2008) The Dolichopodinae (Diptera: Dolichopodidae) of New Caledonia, with descriptions and records from Australia, New Zealand and Melanesia. Zoologica Neocaledonia Vol. 6. (ed. by P. Grandcolas). Mémoires du Muséum national d’Histoire naturelle, 197, 13-47. Bickel, D.J. (2009) Biogeography of Diptera in the southwest Pacific. Diptera diversity: status, challenges tools (ed. by T. Pape, D. Bickel and R. Meier), pp. 1-19. Koninklijke Brill, Leiden. Boon, W.M., Kearvell, J.C., Daugherty, C.H. & Chambers, G.K. (2001) Molecular systematics and conservation of kakariki (Cyanoramphus spp.). Science for Conservation, 176, 1-46. Bouchet, P. & Kantor, Y.I. (2003) New Caledonia: the major centre of biodiversity for volutomitrid molluscs (Mollusca: Neogastropoda: Volutomitridae). Systematics and Biodiversity, 1, 467-502. Bradford, J. (2002) Molecular phylogenetics and morphological evolution in Cunonieae (Cunoniaceae). Annals of the Missouri Botanical Garden, 89, 491-503. Bregulla, H.L. (1992) Birds of Vanuatu. Nelson, Oswestry, Shropshire. Briggs, B.G. & Johnson, L.A.S. (1979) Evolution in the Myrtaceae – evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales, 102, 157-256. Brownlie, G. (1977) The pteridophyte flora of Fiji. (Beihefte zur Nova Hedwigia 55). Cramer, Vaduz. Buckley, T.R. & Simon, C. (2007) Evolutionary radiation of the cicada genus Maoricicada Dugdale (Hemiptera: Cicadoidea) and the origins of the New Zealand alpine biota. Biological Journal of the Linnean Society, 91, 419-435. 48 Burbrink, F.T. (2005) Inferring the phylogenetic positon of Boa constrictor among the Boinae. Molecular Phylogenetics and Evolution, 34, 167-180. Burger, J.F. (1995) The Tabanidae of New Caledonia. Bishop Museum Occasional Papers, 44, 156. Burns, K.C. & Dawson, J.W. 2006. A morphological comparison of leaf heteroblasty between New Caledonia and New Zealand. New Zealand Journal of Botany, 44, 387-396. Burridge, C.P. & Smolenski, A.J. (2004) Molecular phylogeny of the Cheilodactylidae and Latridae (Perciformes: Cirrhitoidea) with notes on taxonomy and biogeography. Molecular Phylogenetics and Evolution, 30, 118-127. Burton, D.W. (1963) A revision of the New Zealand and subantarctic Athoracophoridae. Transactions of the Royal Society of New Zealand, 3, 47-75. Burton, D.W. (1980) Anatomical studies on Australian, New Zealand, and subantarctic Athoracophoridae (Gastropoda: Pulmonata). New Zealand Journal of Zoology, 7, 173-98. Campbell, H.J. (1994) The Triassic bivalves Daonella and Halobia in New Zealand, New Caledonia, and Svalbard. Institute of Geological and Nuclear Sciences Monograph, 4, 1-165. Campbell, H.J. & Grant-Mackie, J.A. (1995) Jurassic Pholadomyidae (Bivalvia) from New Zealand and New Caledonia. New Zealand Journal of Geology and Geophysics, 38, 47-59. Carlquist, S. (1980) Anatomy and systematics of Balanopaceae. Allertonia, 2, 191-246. Carpenter, K.E. & Allen, G.R. (1989) Emperor fishes and large-eye breams of the world (family Lethrinidae). FAO Species Catalogue, 9, 1-118. Chambers, G.K., Boon, W.M., Buckley, T.R. & Hitchmough, R.A. (2001) Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies. Australian Journal of Botany, 49, 377-387. Cheek, M. & Jebb, M. (2001) Nepenthaceae. Flora Malesiana, series 1, 15, 1-157. Chinnock, R.J. (2003) A new species of the genus Tmesipteris in the Society Islands and Marquesas Islands. Appendix. In: Murdock, A.G. and Smith, A.R. Pteridophytes of Moorea, French Polynesia, with a new species, Tmesipteris gracilis (Psilotaceae). Pacific Science, 57, 253-265. Clayton, D. (2002) The genus Coelogyne: a synopsis. Natural History Publications, Kota Kinabalu /Royal Botanic Gardens, Kew, UK. Clifford, H.T., Henderson, R.J.K. & Conran, J.G. (1998) Hemerocallidaceae. The families and genera of vascular plants. Vol. 3. Flowering plants. Monocotyledons: Lilianae (except Orchidaceae) (ed. by K. Kubitzki), pp. 245-253. Springer, Berlin. Conn, B.J. (1980) A taxonomic revision of Geniostoma subgenus Geniostoma (Loganiaceae). Blumea, 26, 245-364. Conran, J.G. (1998) Lomandraceae. In: Kubitzki K, ed. The families and genera of vascular plants. Vol. 3. Flowering Plants. Monocotyledons: Lilianae (except Orchidaceae). Berlin: Springer, 354-365. Coode, M.J.E. (1987) Crinodendron, Dubouzetia and Peripentadenia, closely related in Elaeocarpaceae. Kew Bulletin, 42, 777-814. Corner, E.J.H. (1970) Ficus subgen. Pharmacosycea with reference to the species of New Caledonia. Philosophical Transactions of the Royal Society B: Biological Sciences, 259, 383433. Council of Heads of Australasian Herbaria (2009) Australia’s Virtual Herbarium. http://www.chah.gov.au/avh/ (accessed August 2009). Cowie, R.H. (1992) Evolution and extinction of Partulidae, endemic Pacific island land snails. 49 Philosophical Transactions of the Royal Society B: Biological Sciences, 335, 167-191. Cranston, P.S. (2005) Biogeographic patterns in the evolution of Diptera. The evolutionary biology of flies (ed. by D.K.Yeates and B.M. Wiegmann), pp. 274-311. Columbia University Press, New York. Craven, L. (2001) Unravelling knots or plaiting rope: What are the major taxonomic strands in Syzygium sens. lat. (Myrtaceae) and what should be done with them? Taxonomy: the cornerstone of biodiversity (ed. by L.G. Saw, L.S.L. Chua and K.C. Khoo), 75-85. Institut Penyelidikan Perhutanan Malaysia, Kuala Lumpur. Craven, L., Biffin, E. & Ashton, P.S. (2006) Acmena, Acmenosperma, Cleistocalyx, Piliocalyx and Waterhousea formally transferred to Syzygium (Myrtaceae). Blumea, 51, 131-142. Croizat, L. (1964) Space, time, form: the biological synthesis. Published by the Author, Caracas. Cuccodoro, G. (1998) Revision and phylogeny of Megarthrus Curtis 1829 from New Guinea, New Caledonia and Fiji (Coleoptera Staphylinidae Proteininae). Tropical Zoology, 11, 103-137. Damborenea, S.E. & Manceñido, M.O. (1992) A comparison of Jurassic marine benthonic faunas from South America and New Zealand. Journal of the Royal Society of New Zealand, 22, 131152. Davis, C.C., Bell, C.D., Fritsch, P.W. & Mathews, S. (2002) Phylogeny of AcridocarpusBrachylophon (Malpighiaceae): implications for Tertiary tropical floras and Afroasian biogeography. Evolution, 56, 2395-2405. Davis, C.C., Fritsch, P.W., Bell, C.D. & Mathews, S. (2004) High-latitude Tertiary migrations of an exclusively tropical clade: evidence from Malpighiaceae. International Journal of Plant Sciences, 165, S107-S121. Delacour, J. (1966) Guide des oiseaux de la Nouvelle-Calédonie. Delachaux & Niestlé, Neuchâtel. Diamond, J.M. & Marshall, A.G. (1976) Origins of the New Hebrides avifauna. Emu, 76, 187-200. Dickinson, E.C. (ed.) (2003) The Howard & Moore complete checklist of the birds of the world, 3rd edn. Princeton University Press, Princeton, NJ. Ding Hou & van Steenis, C.G.G.J. (1963) Rhizophora mangle. Pacific Plant Areas, 1, 256-257. Duangjai, S., Wallnöfer, B., Samuel, R., Munzinger, J. & Chase, M.W. (2006) Generic delimitation and relationships in Ebenaceae sensu lato: evidence from six plastid DNA regions. American Journal of Botany, 93, 1808-1827. Duangjai, S., Samuel, R., Munzinger, J., Forest, F., Wallnöfer, B, Barfuss, M.H.J., Fischer, G. & Chase, M.W. (2009) A multi-locus plastid phylogenetic analysis of the pantropical genus Diospyros (Ebenaceae), with an emphasis on the radiation and biogeographic origins of the New Caledonian endemic species. Molecular Phylogenetics and Evolution, 52, 602-620. Duffels, J.P. & Turner, H. (2002) Cladistic analysis and biogeography of the cicadas of the IndoPacific subtribe Cosmopsaltriina (Hemiptera: Cicadoidea: Cicadidae). Systematic Entomology, 27, 235-261. Dumbacher, J.P., Pratt, T.K. & Fleischer, R.C. (2003) Phylogeny of the owlet-nightjars (Aves: Aegothelidae) based on mitochondrial DNA sequence. Molecular Phylogenetics and Evolution, 29, 540-549. Ebihara, A., Iwatsuki, K., Ohsawa, T.A. & Ito, M. (2003) Hymenophyllum paniense (Hymenophyllaceae), a new species of filmy fern from New Caledonia. Systematic Botany, 28, 228-235. Edgecombe, G.D. & Giribet, G. (2004) Molecular phylogeny of Australasian anopsobiine centipedes (Chilopoda: Lithobiomorpha). Invertebrate Systematics, 18, 235-249. Edwards, K.J. & Gadek, P.A. (2001) Evolutionary history of Alectryon in Australia. Faunal and 50 floral migrations and evolution in SE Asia – Australasia (ed. by I. Metcalfe, J.M.B. Smith, M. Morwood and I. Davidson), pp. 244-251. Balkema, Lisse. Eibl, J.M., Plunkett, G.M. & Lowry, P.P., II. (2001) Evolution of Polyscias sect. Tieghemopanax (Araliaceae) based on nuclear and chloroplast DNA sequence data. Adansonia, series 3, 23, 2348. Evans, J.W. (1981) A review of present knowledge of the family Peloridiidae and new genera and new species from New Zealand and New Caledonia (Hemiptera: Insecta). Records of the Australian Museum, 34, 381-406. Evenhuis, N.L. (ed.) (2005a) Catalog of the Diptera of the Australasian and Oceanian Regions. Web version. hbs.bishopmuseum.org/aocat/ (Accessed 2005). Evenhuis, N.L. (2005b) New Cymatopus (Diptera: Dolichopodidae) from Fiji and related areas, with notes on described species. Bishop Museum Occasional Papers, 82, 31-45. Fautin, D.G. & Allen, G.R. (1992) Anemone fishes and their host sea anemones. Western Australian Museum, Perth. Fennah, R.G. (1969) Fulgoroidea (Homoptera) from New Caledonia and the Loyalty Islands. Pacific Insects Monograph, 21, 1-116. Filardi, C.E. & Moyle, R.G. (2005) Single origin of a pan-Pacific bird group and upstream colonization of Australasia. Nature, 438, 216-219. Fisher, K.M. (2006) Rank-free monography: a practical example from the moss clade Leucophanella (Calymperaceae). Systematic Botany, 31, 13-30. Flannery, T.F. (1995) Mammals of the south-west Pacific and Moluccan islands. Sydney: Australian Museum/Reed. Florin, R. (1963) The distribution of conifer and taxad genera in space and time. Acta Horti Bergiani, 21 (4), 121-312. Fricke, R. (2002) Tripterygiid fishes of New Caledonia, with zoogeographical remarks. Environmental Biology of Fishes, 65, 175-198. Frodin, D.G. (2001) Arthrophyllum (Araliaceae) in Malesia. 5th International Flora Malesiana Symposium. Additional Abstracts. Royal Botanic Gardens, Sydney. P. 2. Gadek, P.A, Alpers, D.L., Heslewood, M.M. & Quinn, C.J. (2000) Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. American Journal of Botany, 87, 1044-1057. Gaffney, E.S. (1996) The postcranial morphology of Meiolania platyceps and a review of Meiolaniidae. Bulletin of the American Museum of Natural History, 229, 1-166. Galloway, D.J. (1994) Studies in Pseudocyphellaria (Lichens) IV. Palaeotropical species (excluding Australia). Bulletin of the Natural History Museum London, Botany Series, 24, 115159. Garb, J.E. & Gillespie, R.G. (2006) Island hopping across the central Pacific: mitochondrial DNA detects sequential colonization of the Austral Islands by crab spiders (Araneae: Thomisidae). Journal of Biogeography, 33, 201-220. Gaskin, D.E. (1974) The species of Pareromene Osthelder (Pyralidae: Crambinae: Diptychophorini) from the western South Pacific, with further notes on the New Zealand species. Journal of Entomology (B), 43, 159-184. Gemmill, C.E.C., Allan, G.J., Wagner, W.L. & Zimmer, E.A. (2002) Evolution of insular Pacific Pittosporum (Pittosporaceae): origin of the Hawaiian radiation. Molecular Phylogenetics and Evolution, 22, 31-42. Gibbs, G.W. (1990) Local or global? Biogeography of some primitive Lepidoptera in New 51 Zealand. New Zealand Journal of Zoology, 16, 689-698. Gibbs, G.W. (2006) Ghosts of Gondwana: the history of life in New Zealand. Craig Potton, Nelson. Gill, A.C. & Jewett, S.L. (2004) Eviota hoesi and E. readerae, new species of fish from the southwest Pacific, with comments on the identity of E. corneliae Fricke (Perciformes: Gobiidae). Records of the Australian Museum, 56, 235-240. Givnish, T.J. & Renner, S.S. (2004) Tropical intercontinental disjunctions: Gondwana breakup, immigration from the boreotropics, and transoceanic dispersal. International Journal of Plant Sciences, 165 (4 Suppl.), S1-S6. Glover, E.A. & Taylor, J.D. (2001) Systematic revision of Australian and Indo-Pacific Lucinidae (Mollusca: Bivalvia): Pillucina, Wallucina and descriptions of two new genera and four new species. Records of the Australian Museum, 53, 263-292. Gómez-Zurita, J., Jolivet, P. & Vogler, A.P. (2005) Molecular systematics of Eumolpinae and the relationships with Spilopyrinae (Coleoptera, Chrysomelidae). Molecular Phylogenetics and Evolution, 34, 584-600. González, F. & Rudall, P.J. (2007) Floral morphology of the neotropical family Metteniusaceae, an isolated member of the lamiids. In: Plant Biology and Botany 2007. Program and Abstract Book. Chicago, 192. González, F., Betancur, J., Maurin, O., Freudenstein, J.V. & Chase, M.W. (2007) Metteniusaceae, an early-diverging family in the lamiid clade. Taxon, 56, 795. Good, R. (1950) Madagascar and New Caledonia: a problem in plant geography. Blumea, 6, 470479. Goodwin, D. (1983) Pigeons and doves of the world. 3rd edn. British Museum (Natural History), London/Cornell University Press, New York. Gradstein, S.R. (1975) A taxonomic monograph of the genus Acrolejeunea (Hepaticae).Cramer, Vaduz. Grant-Mackie, J. (1978) Subgenera of the Upper Triassic bivalve Monotis. New Zealand Journal of Geology and Geophysics, 21, 97–111. Grasshoff, M. & Bargibant, G. (2001) Coral reef gorgonians of New Caledonia. Institut de Recherche pour le Développement, Paris. Green, P.S. (1970) Notes relating to the floras of Norfolk and Lord Howe Islands, I. Journal of the Arnold Arboretum, 51, 204-220. Green, P.S. (1972) Passiflora in Australia and the Pacific. Kew Bulletin, 26, 539-558. Green, P.S. (1979) Observations on the phytogeography of the New Hebrides, Lord Howe Island and Norfolk Island. Plants and islands (ed. by D. Bramwell), pp. 41-53. Academic Press, London. Green, P.S. (1990) Notes relating to the floras of Norfolk and Lord Howe Islands, III. Kew Bulletin, 45, 235-255. Green, P.S. (1994) Oceanic islands I. Flora of Australia Vol. 49. Australian Government Publishing Service, Canberra. Gressitt, J.L. (1961) Problems in the zoogeography of Pacific and Antarctic insects. Pacific Insects Monographs, 2, 1-94. Gupta, V.K. (1962) Taxonomy, zoogeography, and evolution of Indo-Australian Theronia (Hymenoptera: Ichneumonidae). Pacific Insects Monograph, 4, 1-142. Haegi, L. & Symon, D.E. (1984) Duboisia. Pacific Plant Areas, 4, 242-243. Hamilton, K.G.A. (1999) The ground-dwelling leafhoppers Myerslopiidae, new family, and 52 Sagmatiini, new tribe (Homoptera: Membracoidea). Invertebrate Taxonomy, 13, 207-235. Harbaugh, D.T. (2007) A taxonomic revision of Australian northern sandalwood (Santalum lanceolatum, Santalaceae). Australian Systematic Botany, 20, 409-416. Harbaugh, D.T. & Baldwin, B.G. (2007) Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. American Journal of Botany, 94, 1028-1040. Harbaugh, D.T., Wagner, W.L., Allan, G.J. & Zimmer, E.A. (2009) The Hawaiian Archipelago is a stepping stone for dispersal in the Pacific: an example from the plant genus Melicope (Rutaceae). Journal of Biogeography, 36, 230-241. Hartley, T.G. (1982) A revision of the genus Sarcomelicope (Rutaceae). Australian Journal of Botany 30, 359-372. Hartley, T.G. (2000) On the taxonomy and biogeography of Euodia and Melicope (Rutaceae). Allertonia, 8, 1-319. den Hartog, C. (1966) Halophila decipiens. Pacific Plant Areas, 2, 216-217. den Hartog, C. (1970) Seagrasses of the world. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, Afdeling Natuurkunde, series 2, 59, 1-275. Heads, M. (2001) Birds of paradise, biogeography and ecology in New Guinea: a review. Journal of Biogeography, 28, 893-927. Heads, M. (2003) Ericaceae in Malesia: vicariance biogeography, terrane tectonics and ecology. Telopea, 10, 311-449. Heads, M. (2006) Panbiogeography of Nothofagus (Nothofagaceae): analysis of the main species massings. Journal of Biogeography, 33, 1066-1075. Heads, M. (2008) Panbiogeography of New Caledonia, south-west Pacific: basal angiosperms on basement terranes, ultramafic endemics inherited from volcanic arcs, and old taxa endemic to young islands. Journal of Biogeography, 35, 2153–2175. Heads, M. (2009a) Globally basal centres of endemism: the Tasman-Coral Sea region (south-west Pacific), Latin America and Madagascar/South Africa. Biological Journal of the Linnean Society, 96, 222-245. Heads, M. (2009b) Vicariance. in: Encyclopedia of Islands (ed. by R.G. Gillespie and D.A. Clague), pp. 947-950. University of California Press, Berkeley. Heads, M. (2009c) Inferring biogeographic history from molecular phylogenies. Biological Journal of the Linnean Society, 98, 757-774. Hennig, W. (1966) Phylogenetic systematics. University of Illinois Press, Urbana. Heraty, J.M. (1995) Classification and evolution of the Oraseminae in the Old World, including revisions of two closely related genera of Eucharitinae (Hymenoptera: Eucharitidae). Royal Ontario Museum Life Sciences Contributions, 157, 1-428. Herbert, D.G. & Mitchell, A. (2008) Phylogenetic relationships of the enigmatic land snail genus Prestonella: the missing African element in the Gondwanan superfamily Orthalicoidea (Mollusca: Stylommatophora). Biological Journal of the Linnean Society,96, 203-221. Herbert, J., Hollingsworth, P.M., Gardner, M.F., Mill, R.R., Thomas, P.I. & Jaffré, T. (2002) Conservation genetics and phylogenetics of New Caledonian Retrophyllum (Podocarpaceae) species. New Zealand Journal of Botany, 40, 175-188. Herman, L.H. (1986) Revision of Bledius. Part 4. Classification of species groups, phylogeny, natural history, and catalogue (Coleoptera, Staphylinidae, Oxytelinae). Bulletin of the American Museum of Natural History, 184, 1-367. Herzer, R.H., Chaproniere, G.C.H., Edwards, A.R., Hollis, C.J., Pelletier, B., Raine, J.I., Scott, 53 G.H., Stagpoole, V., Strong, C.P., Symonds, P., Wilson, G.J. & Zhu, H. (1997) Seismic stratigraphy and structural history of the Reinga Basin and its margins, southern Norfolk Ridge system. New Zealand Journal of Geology and Geophysics, 40, 425-451. van Heusden, E.C.H. (1994) Revision of Meiogyne (Annonaceae). Blumea, 38, 487-511. Hill, K.D. (1994) The Cycas rumphii complex (Cycadaceae) in New Guinea and the western Pacific. Australian Systematic Botany, 7, 543-567. Hill, R.S. & Brodribb, T.J. (1999) Southern conifers in space and time. Australian Journal of Botany, 47, 639-696. Hoeksema, B.W. (1989) Taxonomy, phylogeny, and biogeography of mushroom corals (Scleractinia: Fungiidae). Zoologische Verhandelingen, 254, 1-295. Hoffmann, R.L. (1999) Checklist of the millipedes of North and Middle America. Virginia Museum of Natural History Special Publication, 8, 1-584. Holloway, B.A. (1982) Anthribidae (Insecta: Coleoptera) fauna of New Zealand, 3, 1-264. Holloway, J.D. (1979) A survey of the Lepidoptera, biogeography and ecology of New Caledonia. Junk, The Hague Holloway, J.D. (1993) Lepidoptera in New Caledonia: diversity and endemism in a plant-feeeding insect group. Biodiversity Letters, 1, 92-101. Holloway, J.D. (1996) The Lepidoptera of Norfolk Island, actual and potential, their origins and dynamics. The origin and evolution of Pacific Island biotas, New Guinea to Polynesia: patterns and processes (ed. by A. Keast and S.E. Miller), pp. 123-151. SPB Academic, Amsterdam. Holloway, J.D. & Peters, J.V. (1976) The butterflies of New Caledonia and the Loyalty Islands. Journal of Natural History, 10, 273-318. Holthuis, L.B. (1991) Marine lobsters of the world. FAO Species Catalogue, 13, 1-292. Holttum, R.E. (1964) The tree-ferns of the genus Cyathea in Australasia and the Pacific. Blumea 12, 241-274. Holttum, R.E. (1977) The family Thelypteridaceae in the Pacific and Australasia. Allertonia, 1, 169-234. Holzenthal, R.W. & Oliveira Pes, A.M. (2004) A new genus of long-horned caddisfly from the Amazon basin (Trichoptera: Leptoceridae: Grumichellini). Zootaxa, 621, 1-16. Hoogland, R.D. (1984) Geissois. Pacific Plant Areas, 4, 164-165. Hopkins, H.C.F. (2006) Nomenclature and typification in Geissois (Cunoniaceae) in the SouthWest Pacific. Adansonia, series 3, 28, 311-327. Hopkins, H.C.F. & Bradford, J.C. (1998) A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific. 1. Introduction and an account of the species of Western Malesia, the Lesser Sunda Islands and the Moluccas. Adansonia, series 3, 20, 5-41. Horak, E. (1983) Mycogeography in the South Pacific region: Agaricales, Boletales. Australian Journal of Botany (Supplement Series), 10, 1-41. Houbrick, R.S. (1992) Monograph of the genus Cerithium Bruguière in the Indo-Pacific (Cerithiidae, Prosobranchia). Smithsonian Contributions to Zoology, 510, 1-211. del Hoyo, J., Elliott, A. & Sargatal, J. (1999) Handbook of the birds of the world. Vol. 5. Barnowls to hummingbirds. Lynx, Barcelona. Huber, B.A. (2000) New World pholcid spiders (Araneae: Pholcidae): A revision at generic level. Bulletin of the American Museum of Natural History, 254, 1-348. Hufford, L. & Dickison, W.C. (1992) A phylogenetic analysis of Cunoniaceae. Systematic Botany, 17, 181-200. 54 Hynes, C.D. (1993) The crane-flies of New Caledonia (Diptera Tanyderidae, Tipulidae). Zoologia Neocaledonica vol. 3. (ed. By J. Najt and L. Matile). Mémoires du Muséum National d’Histoire Naturelle, 157, 73-121. Iredale, T. (1912) Concerning the Kermadec Islands avifauna. Transactions of the New Zealand Institute, 45, 78-92. Jacobs, M. (1955) Malpighiaceae. Flora Malesiana, series 1, 5, 125-145. Jacobs, M. & Moore, D.M. (1971) Violaceae. Flora Malesiana, series 1, 7, 179–212. Jaffré, T., Morat, P., Veillon, J.-M., Rigault, F. & Dagostini, G. (2001) Composition et caractérisation de la flore indigène de Nouvelle-Calédonie. (In English and French). Institut de Recherche pour le Développement, Noumea. Jamieson, B.G.M. (1981) Historical biogeography of Australian Oligochaeta. In: Keast A, ed. Ecological biogeography in Australia. The Hague: Junk, 887-922. Jansen, M.E. (1984) Badusa. Pacific Plant Areas, 4, 224-225. Jeekel, C.A.W. (1986) Millipedes from Australia, 10: Three interesting new species and a new genus (Diplopoda: Sphaerotheriida, Spirobolida, Polydesmida). Beaufortia, 36, 35-50. Jennings, J.T. & Austin, A.D. (2002) Systematics and distribution of world hyptiogastrine wasps (Hymenoptera: Gasteruptiidae). Invertebrate Systematics, 16, 735-811. Jessup, L.W. (1982) Flacourtiaceae. Flora of Australia 8, maps 71, 72. Johanson, K.A. & Ward, J.B. (2001) Four new species and a new genus of Trichoptera (Helicophidae) from New Caledonia. New Zealand Journal of Zoology, 28, 247-255. Johns, R.J. & Bellamy, A. (1981) The ferns and fern-allies of Papua New Guinea. Parts 6-12. Research Report of Papua New Guinea University of Technology R 48-81. Johnson, L.A.S. & Briggs, B.G. (1984) Myrtales and Myrtaceae – a phylogenetic analysis. Annals of the Missouri Botanical Garden, 71, 700-756. Jolivet, P., Verma, K.K. & Mille, C. (2007a) New genera and species of Eumolpinae from New Caledonia [Coleoptera, Chrysomelidae]. Revue Française d’Entomologie, 29, 77-92. Jolivet, P., Verma, K.K. & Mille, C. (2007b) New species of Dematochroma from Lord Howe and Norfolk Islands (Coleoptera, Chrysomelidae, Eumolpinae). Nouvelle Revue d’Entomologie, 23, 327-332. Jønsson, K.A., Bowie, R.C.K., Moyle, R.G., Christidis, L., Norma, J.A., Benz, B.W. & Fjeldså, J. (2010) Historical biogeography of an Indo-Pacific passerine bird family (Pachycephalidae): different colonization patterns in the Indonesian and Melanesian archipelagos. Journal of Biogeography, 37, 245-257. Kathriarachchi, H., Samuel, R., Hoffmann, P., Mlinarec, J., Wurdack, K.J., Ralimanana, H., Stuessy, T.F. & Chase, M.W. (2006) Phylogenetics of tribe Phyllantheae (Phyllanthaceae: Euphorbiaceae sensu lato) based on nrITS and matK DNA sequence data. American Journal of Botany, 93, 637-655. Keast, A. (1966) Australia and the Pacific Islands: a natural history. Random House, New York. Knapp, M., Mudaliar, R., Havell, D., Wagstaff, S.J. & Lockhart, P.J. (2007) The drowning of New Zealand and the problem of Agathis. Systematic Biology, 56, 862-870. de Kok, R.P.J. & Mabberley, D.J. (1999) A synopsis of Oxera Labill. (Labiatae). Kew Bulletin, 54, 265-300. Kores, P.J. (1991) Orchidaceae. Flora Vitiensis nova. Vol. 5. (ed. by A.C. Smith), pp. 321575. National Tropical Botanical Garden, Lawai, Kauai, Hawaii. Koster, J.T. (1966) The Compositae of New Guinea I. Nova Guinea, Botany, 24, 497-614. Kostermans, A.J.G.H. (1977) Notes on Asiatic, Pacific, and Australian Diospyros. Blumea, 23, 449-474. 55 Kramina, T. & Sokoloff, D. (2004) A taxonomic study of Lotus australis complex (Leguminosae), with special emphasis on plants from Pacific Ocean islands. Adansonia, series 3, 26, 171-197. Kress, W.J. (1990) The taxonomy of Old World Heliconia (Heliconiaceae). Allertonia, 6, 1-58. Kroenke, L.W. (1996) Plate tectonic development of the Western and Southwestern Pacific: Mesozoic to the present. The origin and evolution of Pacific island biotas, New Guinea to eastern Polynesia: patterns and processes (ed. by A. Keast and S.E. Miller), pp. 19-34. SPB Academic, Amsterdam. Kubitzki, K. (1970) Monographie der Hernandiaceen. Botanische Jahrbücher, 89, 78-148. Kuschel, G. (2008) Curculionoidea (weevils) of New Caledonia and Vanuatu: basal families and some Curculionidae. In: Zoologica Neocaledonia Vol. 6. (ed. by P. Grandcolas). Mémoires du Muséum national d’Histoire naturelle, 197, 99-249. Ladiges, P.Y., Udovicic, F. & Nelson, G. (2003) Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. Journal of Biogeography, 30, 989998. Large, M.F. & Braggins, J.E. (2004) Tree ferns. CSIRO, Melbourne. de Laubenfels, D.J. (1978) The genus Prumnopitys (Podocarpaceae) in Malesia. Blumea, 24, 189190. de Laubenfels, D.J. (1984) Dacrycarpus. Pacific Plant Areas, 4, 210-211. de Laubenfels, D.J. (2005) Statut du Podocarpus de l’Île des Pins (Nouvelle-Calédonie). Adansonia, series 3, 27, 151-153. Leenhouts, P.W. (1955) The genus Canarium in the Pacific. Bulletin of the Bernice P. Bishop Museum, 216, 1-53. Leenhouts, P.W. (1966) Neuburgia. Pacific Plant Areas, 2, 72-73. Léger, N. & Depaquit, J. (2002) Systématique et biogéographie des Phlébotomes (Diptera: Psychodidae). Annales de la Société Entomologique de France, 38, 163-175. Levi, H.W. (1983) The orb-weaver genera Argiope, Gea and Neogea from the western Pacific region (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology, Harvard, 150, 247-338. Levin, R.A., Myers, N.R. & Bohs, L. (2006) Phylogenetic relationships among the ‘spiny solanums’ (Solanum subgenus Leptostemonum, Solanaceae). American Journal of Botany, 93, 157-169. Lewis, B. & Cribb, P. (1989) Orchids of Vanuatu. Royal Botanic Gardens, Kew, UK. Lewis, G., Schrire, B., Mackinder, B. & Lock, M. (2005) Legumes of the world. Royal Botanic Gardens, Kew, UK. Lieftinck, M.A. (1976) The dragonflies (Odonata) of New Caledonia and the Loyalty Islands. Part 1. Imagines. Cahiers O.R.S.T.O.M., sér. Hydrobiologie, 9, 127-166. Lillemets, B. & Wilson, G.D.F. (2002) Armadillidae (Crustacea: Isopoda) from Lord Howe Island: new taxa and biogeography. Records of the Australian Museum, 54, 71-98. Livezey, B.C. (1998) A phylogenetic analysis of the Gruiformes (Aves) based on morphological characters, with an emphasis on the rails (Rallidae). Philosophical Transactions of the Royal Society B: Biological Sciences, 353, 2077-2151. Lorenz, F., Jr, & Hubert, A. (1993) A guide to worldwide cowries. Christa Hemmen, Wiesbaden. Lowry, P.P., II (1989) A revision of Araliaceae from Vanuatu. Bulletin du Muséum National d’Histoire Naturelle [Paris], 4th series 11, sect. B, Adansonia, 2, 117-155. Lowry, P.P., II (1998) Diversity, endemism, and extinction in the flora of New Caledonia: a Review, Rare, threatened, and endangered floras of Asia and the Pacific rim (Academica Sinica 56 Monograph 16) (ed. by C.I. Peng and P.P. Lowry II), pp. 181-206. Institute of Botany, Taipei. Lu, W. & Wang, Q. (2005) Systematics of the New Zealand longicorn beetle genus Ophyrops White (Coleoptera: Cerambycidae: Cerambycinae). Invertebrate Systematics, 19, 169-188. Mabberley, D.J. (1998) On Neorapinia (Vitex sensu lato, Labiate-Viticoideae). Telopea, 7, 313317. Maciolek, J.A. (1983) Distribution and biology of Indo-Pacific insular hypogeal shrimps. Bulletin of Marine Science, 33, 606-618. Mackerras, I.M. (1970) Composition and distribution of the fauna. The insects of Australia (ed. by I.M. Mackerras), pp. 187-203. Melbourne University Press, Melbourne Mackerras, I.M. (1971) Papuan-Melanesian Diachlorini (Diptera: Tabanidae). Pacific Insects, 13, 405-427. Malm, T. & Johanson, K.A. (2008) Revision of the New Caledonian endemic genus Gracilopsodes (Trichoptera: Leptoderidae: Grumichellini). Zoological Society of the Linnean Society, 153, 425452. Manconi, R. & Serusi, A. (2008) Rare sponges from marine caves: discovery of Neophrissospongia nana nov. sp. (Demospongiae, Corallistidae) from Sardinia with an annotated checklist of Mediterranean lithistids. ZooKeys, 4, 71-87. Markgraf, F. (1977) Florae Malesianae praecursores LV. Apocynaceae IV. Alyxia. Blumea, 23, 377-414. Martens, K. & Rossetti, G. (2002) On the Darwinulidae (Crustacea: Ostracoda) from Oceania. Invertebrate Systematics, 16, 195-208. Matthews, E.G. (1998) Classification, phylogeny and biogeography of the genera of Adeliini (Coleoptera: Tenebrionidae). Invertebrate Taxonomy, 12, 685-824. Mayr, E. (1933) Birds collected during the Whitney South Sea Expedition. XXII. Three new genera from Polynesia and Melanesia. American Museum Novitates, 590, 1-6. Mayr, E. & Diamond, J. (2001) The birds of northern Melanesia: speciation, ecology and biogeography. Oxford University Press, New York. McKenna, M.C. (1983) Holarctic land mass rearrangement, cosmic events and Cenozoic terrestrial organisms. Annals of the Missouri Botanical Garden, 70, 459-489. McPherson, G. & Lowry, P.P., II (2004) Hooglandia, a newly discovered genus of Cunoniaceae from New Caledonia. Annals of the Missouri Botanic Garden, 91, 260-265. van Meerendonk, J.P.M. (1984) Triuridaceae. Flora Malesiana, series 1, 10, 109-121. Mesibov, R. (2009) Revision of Agathodesmus Silvestri, 1910 (Diplopoda, Polydesmida, Haplodesnidae). ZooKeys, 12, 87-110. Michaux, B. & White, I.M. (1999) Systematics and biogeography of southwest Pacific Bactrocera (Diptera: Tephritidae: Dacini). Palaeogeography, Palaeoclimatology, Palaeoecology, 153, 337351. Middleton, D.J. (2002) Revision of Alyxia (Apocynaceae). Part 2: Pacific Islands and Australia. Blumea, 47, 1-93. Millar, A.J.K. & Bolton, J.J. (2004) Sharing marine algae – Australia and South Africa. Abstract. In: Southern temperate ecosystems and biota: contributions towards a global synthesis. Programme and Abstracts. Cape Town: University of Cape Town. Published at: web.uct.ac.za/ conferences/sc2004/ Mille, C. (2008) Fruit flies in New Caledonia. Zoologica Neocaledonia Vol. 6 (ed. by P. Grandcolas).Mémoires du Muséum national d’Histoire naturelle, 197, 251-259. Miller, H.A., Whittier, H.O. & Whittier, B.A. (1978) Prodromus florae Muscorum Polynesiae: 57 with a key to genera. Vaduz: Cramer. Miller, H.A., Whitter, H.O. & Whittier, B.A. (1983) Prodromus florae Hepaticarum Polynesiae: with a key to genera. Cramer, Vaduz. Molvray, M. (1997) A synopsis of Korthalsella (Viscaceae). Novon, 7, 268-273. Monteith, G.B. (1979) Relationships of the genera of Chinamyersiinae, with descriptions of a relict species from mountains of north Queensland (Hemiptera: Heteroptera: Aradidae). Pacific Insects, 31, 275-285. Mooi, R.D. (1995) Revision, phylogeny and discussion of biology and biogeography of the fish genus Plesiops (Perciformes: Plesiopidae). Royal Ontario Museum Life Sciences Contributions, 159, 1-108. Morat, P., Veillon, J.-M. & MacKee, H.S. (1984) Floristic relationships of New Caledonian rain forest phanerogams. Biogeography of the tropicalPacific (ed. by P. Raven, F. Radovsky and S. Sohmer), pp. 71-128. Association of Systematics Collections and Bernice P. Bishop Museum, Honolulu. Morat, P., Deroin, T. & Couderc, H. (1994) Présence en Nouvelle-Calédonie d’une espèce endémique du genre Oryza L. (Gramineae). Bulletin du Muséum National d’Histoire Naturelle B, Adansonia 16, 3-10. Morat, P., Jaffré, T. & Veillon, J.-M. (2001) The flora of New Caledonia’s calcareous substrates. Adansonia, series 3, 23, 109-127. Morrone, J.J. (2002) Biogeographical regions under track and cladistic scrutiny. Journal of Biogeography, 29, 149-152. Motley, T.J., Wurdack, K.J. & Delprete, P.G. (2005) Molecular systematics of the CatesbaeeaeChiococceae complex (Rubiaceae): flower and fruit evolution and biogeographic implications. American Journal of Botany, 92, 316-329. Moyle, R.G., Filardi, C.E., Smith, C.E. & Diamond, J. (2009) Explosive Pleistocene diversification and hemispheric expansion of a ‘great speciator’. Proceedings of the National Academy of Sciences USA, 106, 1863-1868. Muona, J. (1991) The Eucnemidae of South-east Asia and the Western Pacific – a biogeographical study. Australian Systematic Botany, 4, 165-182. Naczi, R.F.C., Reznicek, A.A. & Ford, B.A. (1998) Morphological, geographical, and ecological differentiation in the Carex willdenowii complex (Cyperaceae). American Journal of Botany, 85, 434-447. Nesom, G.L. (2001) Taxonomic notes on Keysseria and Pytinicarpa (Asteraceae: Astereae: Lageniferinae). Sida, 19, 513-518. Newton, A. (2004) Proteininae (Insecta: Coleoptera: Staphylinidae) in Gondwana: first look at ancient distribution patterns. Abstract. In: Southern temperate ecosystems and biota: contributions towards a global synthesis. Programme and Abstracts. Cape Town: University of Cape Town. Published at: web.uct.ac.za/conferences/sc2004/ Nowak, R. (1999) Walker's mammals of the world. Baltimore: Johns Hopkins University Press. Oginuma, K., Munzinger, J. & Tobe, H. (2006) Exceedingly high chromosome number in Strasburgeriaceae, a monotypic family endemic to New Caledonia. Plant Systematics and Evolution, 262, 97-101. Opell, B.D. (1983) A review of the genus Tangaroa (Araneae, Uloboridae). Journal of Arachnology, 11, 287-295. Ormerod, P. & Cribb, P. (2003a) Danhatchia and Gonatostylis. Genera Orchidacearum. Vol. 3. Orchidoideae (Part 2).Vanilloideae (ed. by A.M. Pridgeon, P.J. Cribb, M.W. Chase and F.N. 58 Rasmussen), pp. 82-85, 90-94. Oxford University Press, New York. Ormerod, P. & Cribb, P. (2003b) Erythrodes and Microchilus. Genera Orchidacearum. Vol. 3. Orchidoideae (Part 2). Vanilloideae (ed. by A.M. Pridgeon, P.J. Cribb, M.W. Chase and F.N. Rasmussen), 85-90, 121-124. Oxford University Press, New York. Page, C.N. (1980) Leaf micromorphology in Agathis and its taxonomic implications. Plant Systematics and Evolution, 135, 71-79. Page, T.J., Baker, A.M., Cook, B.D. & Hughes, J.M. (2005) Historical transoceanic dispersal of a freshwater shrimp: the colonization of the South Pacific by the genus Paratya (Atyidae). Journal of Biogeography, 32, 581-593. Page, T.J., von Rintelen, K. & Hughes, J.M. (2007) An island in the stream: Australia’s place in the cosmopolitan world of Indo-West Pacific freshwater shrimp (Decapoda: Atyidae: Caridina). Molecular Phylogenetics and Evolution, 43, 645-659. Pamaby, H.E. (2002) A new species of long-eared bat (Nyctophilus: Vespertilionidae) from New Caledonia. Australian Mammalogy, 23, 115-124. Payens, J.P.D.W. (1967) A monograph of the genus Barringtonia (Lecythidaceae). Blumea, 15, 157-263. Perrie, L.R, Bayly, M.J., Lehnebach, C.A. & Brownsey, P.J. (2007) Molecular phylogenetics and molecular dating of the New Zealand Gleicheniaceae. Brittonia, 59, 129-141. Philipson, W.R. (1979) Araliaceae – I. Flora Malesiana, series 1, 9, 1-105. Philipson, W.R. (1986) Trimeniaceae. Flora Malesiana, series 1, 10, 327-333. Phillimore, A.B. (2006) The ecological basis of speciation and divergence in birds. Unpublished PhD Thesis, University of London. Pillon, Y. (2008) Biodiversité, origine et evolution des Cunoniaceae: implications pour la conservation de la flore de Nouvelle-Calédonie. Unpublished PhD Thesis, Université de la Nouvelle-Calédonie. Pillon, Y., Hopkins, H.C.F., Munzinger, J. & Chase, M.W. (2009) A molecular and morphologival survey of generic limits of Acsmithia and Spiraeanthemum (Cunoniaceae). Systematic Botany, 34, 141-148. Platnick, N.I. (2007) The world spider catalog, version 5.5. Available at: research.amnh.org/entomology/spiders/catalog/ (Accessed May 2007). Plunkett, G.M., Lowry, P.P., II & Burke, M.K. (2001) The phylogenetic status of Polyscias (Araliaceae) based on nuclear ITS sequences. Annals of the Missouri Botanical Garden, 88, 213-230. Plunkett, G.M., Wen, J. & Lowry, P.P., II (2004) Intrafamilial classifications and characters in Araliaceae: insights from the phylogenetic analysis of nuclear (ITS) and plastid (trnL-trnF) sequence data. Plant Systematics and Evolution, 245, 1-39. Plunkett, G.M., Lowry, P.P., II, Frodin, D.G. & Wen, J. (2005) Phylogeny and biogeography of Schefflera: pervasive polyphyly in the largest genus of Araliaceae. Annals of the Missouri Botanic Garden, 92, 202-224. Pole, M. (2007) Plant-macrofossil assemblages during Pliocene uplift, South Island, New Zealand. Australian Journal of Botany, 55, 118–142. Pole, M. (2009) Was New Zealand a primary source for the New Caledonian flora? Alcheringa DOI: 10.1080/03115510903343477. Polhemus, D.A., Englund, R.A., Allen, G.R., Boseto, D. & Polhemus, J.T. (2008) Freshwater biotas of the Solomon Islands: analysis of richness, endemism and threats. Bishop Museum Technical Report, 45, 1-102. 59 Ponder, W.F. (1983) A revision of the recent Xenophoridae of the world and of the Australian fossil species (Mollusca: Gastropoda). Memoirs of the Australian Museum, 17, 1-126. Ponder, W.F. (2003) Monograph of the Australian Bithyniidae (Caenogastropoda: Rissoidea). Zootaxa, 230, 1-126. Ponder, W.F. & Yoo, E.K. (1980) A review of the genera of the Cingulopsidae with a revision of the Australian species. Records of the Australian Museum, 331, 1-88. Poon, W.-S., Shaw, P.-C., Simmons, M.P. & But, P. P.-H. (2007) Congruence of molecular, morphological, and biochemical profiles in Rutaceae: a cladistic analysis of the subfamilies Rutoideae and Toddalioideae. Systematic Botany, 32, 837-846. Prance, G.T. (1979) New genera and species of Chrysobalanaceae from Malesia and Oceania. Brittonia 31, 79-95. Pulvers, J.N. & Colgan, D.J. (2007) Molecular phylogeography of the fruit bat genus Melonycteris in northern Melanesia. Journal of Biogeography, 34, 713-723. Radcliffe-Smith, A. (2001) Genera Euphorbiacearum. Royal Botanic Gardens, Kew, UK. Randall, J.E. & Heemstra, P.C. (1991) Revision of Indo-Pacific Groupers (Perciformes: Serranidae: Epinephelinae), with descriptions of five new species. Indo-Pacific Fishes, 20, 1-332. Renner, S.S., Murray, D. & Foreman, D. (2000) Timing transantarctic disjunctions in the Atherospermataceae (Laurales): evidence from coding and noncoding chloroplast sequences. Systematic Biology, 49, 579-591. Renz, J. & Vodonaivalu, S. (1989) Studies in the Subtribe Habenariinae (Orchidaceae) IV. Habenaria, Peristylus, and Cynorkis from the Fiji Islands. Blumea, 34, 87-98. Richardson, J.E., Weitz, F.M., Fay, M.F., Cronk, Q.C.B., Linder, P.H., Reeves, G. & Chase, M.W. 2001. Rapid and recent origin of species richness in the Cape flora of South Africa. Nature, 412, 181-183. Richer de Forges, B. (1993) Deep sea crabs of the Tasman Seamounts (Crustacea: Decapoda: Brachyura). Records of the Australian Museum, 45, 11-24. Richer de Forges, B. & Ng, P.K.L. (2009) New genera, new species and new records of Indo-West Pacific spider crabs (Crustacea: Brachyura: Epialridae: Majoidea) Zootaxa, 2025, 1-20. Richer de Forges, B., Koslow, J.A. & Poore, G.C.B. (2000) Diversity and endemism of the benthic seamount fauna in the southwest Pacific. Nature, 405, 944-947. Richling, I. (2009) The radiation of the Helicinidae in New Caledonia (Mollusca: Gastropoda: Neritopsina) including zoogeographic considerations. Zoologia Neocaledonica Vol. 7. Biodiversity studies in New Caledonia (ed. by P. Grandcolas). Mémoires du Muséum national d’Histoire naturelle, 198, 247-372. Rintz, R.E. (1980) A revision of the genus Sarcolobus (Asclepiadaceae). Blumea, 26, 65-79. Ripley, S.D. (1977). Rails of the world: a monograph of the family Rallidae. Toronto, Feheley. Robson, N.K.B. (1981) Studies in the genus Hypericum L. (Guttiferae) 2. Characters of the genus. Bulletin of the British Museum (Natural History) (Botany), 8, 55-226. Röckel, D., Korn, W. & Kohn, A.J. (1995) Manual of the living Conidae. Vol. 1. Indo-Pacific region. Christa Hemmen, Wiesbaden. Roig-Juñent, S. (2000) The subtribes and genera of the tribe Broscini (Coleoptera: Carabidae): cladistic analysis, taxonomic treatment, and biogeographical considerations. Bulletin of the American Museum of Natural History, 255, 1-90. Roisin, Y. & Pasteels, J.M. (1996) The nasute termites (Isoptera: Nasutitermitinae) of Papua New Guinea. Invertebrate Taxonomy, 10, 507-616. 60 Sastre-De Jesús, I. (1987) Revision of the Cyrtopodaceae and transfer of Cyrtopodendron to the Pterobryaceae. Memoirs of the New York Botanical Garden, 45, 709-721. Sauer, J. (1964) Revision of Canavalia. Brittonia, 16, 106-181. Schmalfuss, H. (2009) World catalog of terrestrial isopods (Isopoda: Oniscidea). http://www.naturkundemuseum-bw.de/stuttgart/projekte/oniscidea-catalog/Cat_terr_isop.pdf Schuh, R.T. (1984) Revision of the Phylinae (Hemiptera, Miridae) of the Indo-Pacific. Bulletin of the American Museum of Natural History, 177, 1-462. Schuster, R.M. (1969) Problems of antipodal distribution in lower land plants. Taxon, 18, 46-91. Schuster, R.M. (1979) On the persistence and dispersal of transantarctic Hepaticae. Canadian Journal of Botany, 57, 2179-2225. Schuster, R.M. (1982) Generic and familial endemism in the hepatic flora of Gondwanaland: origins and causes. Journal of the Hattori Botanical Laboratory, 52, 3-35. Schuster, R.M. (1984) Phytogeography of the Bryophyta. In:, ed. New manual of bryology (ed. by R.M. Schuster), pp. 463-626. Hattori Botanical Laboratory, Nichinan, Miyazaki, Japan. Scott, A.J. (1978) A revision of Rhodomyrtus (Myrtaceae). Kew Bulletin, 33, 311-329. Scott, A.J. (1979a) A revision of Xanthomyrtus (Myrtaceae). Kew Bulletin, 33, 461-484. Scott, A.J. (1979b) New species and combinations in the Myrtaceae from Malesia and Ausralia. Kew Bulletin, 33, 511-515. Scott, A.J. (1980) Notes on Myrtaceae in the Mascarenes with some recombinations for taxa from Aldabra, Malaya, New Caledonia. Kew Bulletin, 34, 473-498. Selling, O.H. (1946) Two new species of Schizaea and their affinities. Svensk Botanisk Tidskrift, 40, 273-283. Setoguchi, H., Osawa, T.A., Pintaud, J.-C., Jaffré, T. & Veillon, J.-M. (1998) Phylogenetic relationships within the Araucariaceae on rbcL gene sequences. American Journal of Botany, 85, 1507-1506. Sidiyasa, K. (1998) Taxonomy, phylogeny, and wood anatomy of Alstonia (Apocynaceae). Blumea Supplement, 11, 1-230. Sipman, H.J.M. (1983) A monograph of the lichen family Megalosporaceae. [Mededelingen van het Botanisch Museum en Herbarium van de Rijksuniversiteit te Utrecht, Vol. 482]. Cramer, Vaduz. Skottsberg, C. (1937) Recent researches in Astelia B. and S. Transactions of the Royal Society of New Zealand, 67, 218-226. Smith, A.C. (1978) A precursor to a new flora of Fiji. Allertonia, 1, 331-414. Smith, A.C. (1979-1996). Flora Vitiensis nova: a new flora of Fiji (spermatophytes only). 6 vols. Pacific Tropical Botanical Garden, Lawai, Kauai, Hawaii. So, M.L. (2000) Studies on Plagiochila (Hepaticae) in Australasia and the Pacific II. Cryptogamie Bryologie, 21, 223-231. So, M.L. (2002) The genus Porella (Porellaceae, Hepaticae) in Australasia and the Souith Pacific. Systematic Botany, 27, 4-13. So, M.L. (2003) Schistochila (Hepaticae) in Oceania. New Zealand Journal of Botany, 41, 255275. So, M.L. & Grolle, R. (2001) On Plagiochila subgenus Plagiochila section Abietinae (Hepaticae). Systematic Botany, 26, 459-469. Solem, A. (1958) Biogeography of the New Hebrides. Nature, 181, 1253-1255. Springer, V.G. (1982) Pacific plate biogeography, with special reference to shorefishes. 61 Smithsonian Contributions to Zoology, 367, 1-182. Springer, V.G. & Williams, J.T. (1994) The Indo-West Pacific blenniid fish genus Istiblennius reappraised: a revision of Istiblennius, Blenniella, and Paralticus, new genus. Smithsonian Contributions to Zoology, 565, 1-193. Springer, V.G. (1999) Ecsenius polystictus, new species of blenniid fish from Mentawai Islands, Indonesia, with notes on other species of Ecsenius. Revue Français Aquariologie, 16, 39-48. Springer, V.G. (2002) Ecsenius niue, new species of blenniid fish, and new distribution records for other species in the Opsifrontalis species group. Zootaxa, 72, 1-6. Steadman, D.W. (2006) Extinction and biogeography of tropical Pacific birds. University of Chicago Press, Chicago. Steane, D.A., Wilson, K.L. & Hill, R.S. (2003) Using matK sequence data to unravel the phylogeny of Casuarinaceae. Molecular Phylogenetics and Evolution, 28, 47-59. van Steenis, C.G.G.J. (1963) Dolichandrone spathacea. Pacific Plant Areas, 1, 248-249. van Steenis, C.G.G.J. (1977) Bignoniaceae. Flora Malesiana, series 1, 8, 114-186. Stemmerik, J.F. (1964) Florae Malesianae praecursores XXXVIII. Notes on Pisonia L. in the Old World (Nyctaginaceae). Blumea, 12, 275-284. Stevens, P.F. Accessed 2009. Angiosperm phylogeny website. Version 9. www.mobot.org/MOBOT/Research/APweb/welcome.html Stilwell, J.D. (1994) The paleaoaustral genus Protodolium Wilckens, 1922 (Mollusca: Gastropoda), and a new species from the Late Cretaceous of Chatham Islands, New Zealand. Journal of the Royal Society of New Zealand, 24, 1-16. St. John, H. (1965) Revision of Capparis spinosa and its African, Asiatic, and Pacific relatives. Micronesica, 2, 25-44. Stone, B.C. (1973) Materials for a monograph of Freycinetia Gaudich. XIV. On the relation between F. banksii A.Cunn. of New Zealand and F. baueriana Endl. of Norfolk Island, with notes on the structure of the seed. New Zealand Journal of Botany, 11, 241-246. Stone, B.C. (1981) New Guinea Pandanaceae: first approach to ecology and biogeography. Biogeography and ecology of New Guinea (ed. by J.L. Gressitt), pp. 401-436. Junk, The Hague. Swenson, U., Bartish, I.V. & Munzinger, J. (2007) Phylogeny, diagnostic characters and generic limitation of Australasian Chrysophylloideae (Sapotaceae, Ericales): evidence from ITS sequence data and morphology. Cladistics, 23, 201-228. Swenson, U., Munzinger, J. & Bartish, I.V. (2007) Molecular phylogeny of Planchonella (Sapotaceae) and eight new species from New Caledonia. Taxon, 56, 329-354. Takahashi, H. (1998) Mycena auricoma, a new species of Mycena section Radiatae from Japan, and Mycena spinosissima, a new record from Japan. Mycoscience, 40, 73-80. Takhtajan, A. (1986) Floristic regions of the world. University of California Press, Berkeley. Tangney, R. (1990) Moss biogeography in the Tasman Sea region. New Zealand Journal of Zoology, 16, 665-678. Taylor, B. & Maffi, M. (1978) A review of the mosquito fauna of the Solomon Islands (Diptera: Culicidae). Pacific Insects, 19, 165-248. Thacker, C.E. & Hardman, M.A. (2005) Molecular phylogeny of baal gobioid fishes: Rhyacichthyidae, Odontobutidae, Xenisthmidae, Eleotridae (Teleostei: Perciformes: Gobioidei). Molecular Phylogenetics and Evolution, 37, 858-871. Thorne, R.F. (1965) Floristic relationships of New Caledonia. University of Iowa Studies in Natural History, 20 (7), 1-14. Thorne, R.F. (1969) Floristic relationships between New Caledonia and the Solomon Islands. 62 Proceedings of the Royal Society B: Biological Science, 255, 595-602. Thornton, I.W.B. (1981) Psocoptera of the Fiji Islands. Pacific Insects Monographs, 37, 1-105. Tixier, P. (1979) Mosses from Fiji, New Caledonia, Samoa and Society Islands, collected by H.S. MacKee (Bryophyta exotica IV). Nova Hedwigia, 31, 693-719. Tronchet, F., Plunkett, G.M., Jérémie, J. & Lowry, P.P., II (2005) Monophyly and major clades of Meryta (Araliaceae). Systematic Botany, 30, 657-670. Valdés, Á. (2001a) Depth-related adaptations, speciation processes and evolution of color in the genus Phyllidiopsis (Mollusca: Nudibranchia). Marine Biology, 139, 485-496. Valdés, Á. (2001b) Deep-water phyllidiid nudibranchs (Gastropoda: Phyllidiidae) from the tropical south-west Pacific Ocean. Tropical deep-sea benthos, Vol. 22 (ed. by P. Bouchet and B.A. Marshall). Mémoires du Muséum National d’Histoire Naturelle, 185, 331-368. Veron, J.E.N. (2000) Corals of the world. 3 vols. Australian Institute of Marine Science, Townsville. Vink, W. (1985) The Winteraceae of the Old World V. Exospermum links Bubbia to Zygogynum. Blumea, 31, 39-55. de Vogel, E.F. (1975) Prosopis insularum. Pacific Plant Areas, 3, 312-313. Vuilleumier, F. & Gochfeld, M. (1976) Notes sur l’avifaune de Nouvelle-Calédonie. Alauda, 44, 237-273. Wagstaff, S.J. & Dawson, M.J. (2000) Classification, origin, and patterns of diversification of Corynocarpus (Corynocarpaceae) inferred from DNA sequences. Systematic Botany, 25, 134149. Wattel, J. (1973) Geographical differentiation in the genus Accipiter. Publications of the Nuttall Ornithological Club, 13, 1-231. Webster, G.L. (1986) A revision of Phyllanthus (Euphorbiaceae) in eastern Melanesia. Pacific Science, 40, 88-105. Webster, G.L. & Airy Shaw, H.K. (1971) A provisional synopsis of the New Guinea taxa of Phyllanthus (Euphorbiaceae). Kew Bulletin, 26, 85-109. Weir, A.A.S., Kenward, B., Chappell, J. & Kacelnik, A. (2004) Lateralization of tool use in New Caledonian crows (Corvus moneduloides). Proceedings of the Royal Society B: Biological Science (Supplement), 271, S344-S346. Westermann, G.E.G. (2000) Marine faunal realms of the Mesozoic: review and revision under the new guidelines for biogeographic classification and nomenclature. Palaeogeography, Palaeoclimatology, Palaeoecology, 163, 49-68. Weston, P.H. & Barker, N.P. (2006) A new suprageneric classification of the Proteaceae, with an annotated checklist of genera. Telopea, 11, 314-344. Whitehead, P.J.P. (1985) Clupeioid fishes of the world. Part 1. Chirocentridae, Clupeidae and Pristigasteridae. FAO Species Catalogue, 7, 1-301. de Wilde, W.J.J.O. (1972) Passifloraceae. Flora Malesiana, series 1, 7, 405-434.Williams, D.J. & Watson, G.W. (1988) The scale insects of the tropical Pacific region. Part 1. The armoured scales (Diaspididae). C.A.B. International Institute of Entomology, Wallingford, Oxon. Willis, C.L. (2007) Inference of phylogenetic relationships in Macadamia and relatives (tribe Macadamieae; Proteaceae) using three chloroplast and three nuclear DNA regions. Unpublished Honors Degree Thesis, Florida State University. Available at: http://dscholarship.lib.fsu.edu/ (Accessed October 2009). Wilmot-Dear, C.M. & Friis, I. (1998) Cypholophus decipiens (Urticaceae) – taxonomy and range 63 of a species often misplaced in Boehmeria. Kew Bulletin, 53, 919-927. Wilson, P.G. (1996) Myrtaceae in the Pacific, with special reference to Metrosideros. The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: patterns and processes (ed. by A. Keast and S.E. Miller), pp. 233-245. SPB Academic Publishing, Amsterdam. Wilson, P.G., O’Brien, M.M., Heslewood, M.M. & Quinn, C.J. (2005) Relationships within Myrtaceae sensu lato based on a matK sequence. Plant Systematics and Evolution, 251, 3-19. Wilson, P.G., Heslewood, M.M. & Quinn, C.J. (2007) Re-evaluation of the genus Babingtonia (Myrtaceae) in eastern Australia and New Caledonia. Australian Systematic Botany 20, 302-318. Worthy, T.H. (2004) The fossil rails (Aves: Rallidae) of Fiji with descriptions of a new genus and species. Journal of the Royal Society of New Zealand, 34, 295-314. Worthy, T.H., Miskelly, C.M. & Ching, R.A. (2002) Taxonomy of North and South Island snipe (Aves: Scolopacidae: Coenocorypha), with analysis of a remarkable collection of snipe bones from Greymouth, New Zealand. New Zealand Journal of Zoology, 29, 231-244. Wright, S.E.D., Yong, C.G., Dawson, J.W., Whittaker, D.J. & Gardner, R.C. (2000) Riding the ice age El Niño? Pacific biogeography and evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proceedings of the National Academy of Science USA, 97, 4118-4123. Zug, G.R. (1991) Lizards of Fiji: natural history and systematics. Bishop Museum Press, Honolulu. 64