AUTONOMIC CONTROL OF BLOOOD PRESSURE
advertisement

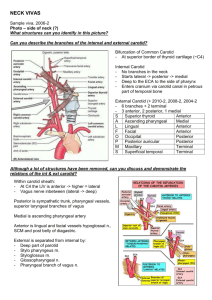
AUTONOMIC CONTROL OF BLOOOD PRESSURE BACKGROUND In this experiment you will study reflex circuits that are part of a negative feedback system that controls arterial blood pressure. The pressure receptors for the afferent side of this circuit are located in the walls of the aortic arch and in the walls of the carotid sinus, an enlargement of the carotid artery at the point where the common carotid branches to form the internal and external carotids. The afferent axons of the carotid baroreceptors course in cranial n. IX, the glossopharyngeal nerve. Those from the aorta course in cranial n. X, the vagus nerve. The efferent limb of this feedback loop is formed by sympathetic nerves to the vasculature and by the parasympathetic and sympathetic innervation of the heart. The sheath surrounding the carotid artery encloses three nerves: the main trunk of the vagus, a branch of the vagus termed the aortic depressor branch, and the cervical sympathetic nerve. The aortic depressor branch contains afferents from the baroreceptors and some chemoreceptors in the aortic arch and common carotid arteries. The branch can be identified by electrical stimulation: the main trunk of the vagus should give dramatic slowing of the heart while the depressor will produce a drop in blood pressure with a less marked decrease in heart rate. Note: in some rabbits the depressor branches off in the thorax instead of high in the neck; if you get one of these animals you will not be able to carry out all parts of the experiment. Dissect carefully since the depressor may be tiny and lie close to the main vagus. Consult the instructor before you conclude that there is no depressor branch. SURGICAL PROCEDURE 1. Anesthetize a rabbit and shave the neck region. 2. Make the neck incision. When you have cut through the skin, put the scalpel aside and proceed to expose the trachea by blunt dissection. 3. Choose a tracheal cannula of appropriate size. Pass two ligatures under the trachea. Pass a curved hemostat under the trachea and above it make a transverse cut part way through the trachea. Clean up any blood in the airway with a cotton swab. Insert the cannula, and tie it in securely with the ligatures. 4. Find the jugular vein in the far side of the animal's neck and clean away the surrounding fat and connective tissue using blunt dissection methods. Have the I.V. drip line filled and free of air bubbles. Pass two threads under the jugular. Clamp the jugular at the tail end using a small serrifine clamp. Use one thread to tie off the jugular at the head end. Make a hole in the vein using a 21-gauge needle. Insert the drip line pointing toward the heart and tie it in securely. Remove the clamp and check for leaks. Start the drip going at about 10 drops/minute. 5. Carefully isolate the vagi and depressors on both sides of the neck. Place loose ligatures around the nerves as indicated below, using different colors of thread for the depressor and vagus on each side: 2 around the right vagus 1 around the left vagus 2 around the left depressor 1 around the right depressor 6. Clean the carotid on the side of the neck nearest to you. Have the carotid cannula ready to insert by flushing out all bubbles in the system and calibrating the blood pressure transducer according to the instructions on top of the amplifier. The computer should be set up to record one channel. Pass two threads under the carotid. Tie the vessel off at the head end. Clamp it at the tail end. Make a hole using a 21-gauge needle. Insert the carotid cannula toward the heart. Tie it in securely and remove the clamp. Check for leaks. Take care not to damage the nerves in the process. EXPERIMENTAL PROCEDURE 1. Collect 1 or 2 screens of cardiac activity for a baseline record. 2. To show the effect of epinephrine, plan to collect data for 30 sec. starting with the injection of epinephrine. Then collect another 30 sec record after a 1-minute delay. Then collect a third record after 3 minutes. When you are organized to do this, inject 0.20 ml of 1/10,000 epinephrine via the IV line. The anesthetist should monitor the state of the animal carefully during this time. If the animal stops breathing, you must immediately ventilate him by hand or with the respirator. 3. Collect another baseline record after the animal has recovered from the effects of the epinephrine. 4. Place a serrifine clamp on the carotid artery that has not been cannulated. For this step, collect a 30 second record starting when the clamp is applied and another one starting when the clamp is removed. What effect do you see on blood pressure and heart rate? Remove the clamp and watch for recovery. 5. Set the stimulator to deliver "burst" stimulation at a frequency of 100/sec and a duration of 1 msec. Set the voltage to the lowest value (0.1 volt). Lift the left depressor free of blood and tissue and lay it on the stimulating electrodes. Increase the voltage until a clear response is seen (or until you conclude that you have the wrong nerve). Be ready to save data when you get a good response. 6. Tighten the two ligatures around the left depressor nerve and cut it between the ligatures. Stimulate the cephalic stump. What response do you get? 7. Stimulate the caudal stump in the same way. Do you get a response? 8. Verify that the presumed right depressor really is the right depressor by stimulating it. Then position the ligature as far caudally as possible, tighten the ligature and cut the nerve just caudal to the ligature. Now both depressors are cut. 9. Again place a serrifine clamp on the carotid. Is the response stronger or weaker? 10. Tighten the two ligatures around the right vagus near the middle of its exposed portion. Sever the right vagus between the ligatures. Note any effect on respiration and blood pressure. 11. Set the stimulator to a low voltage. Stimulate the caudal end of the right vagus. Raise the voltage until you get a response. Record the voltage. What is the effect on blood pressure? 12. Stimulate the cephalic end of the right vagus. Is there an effect on respiration? Is there an effect on blood pressure? Why? Remember the vagus contains visceral afferents as well as efferents. 13. Stimulate the cephalic stump of the right depressor nerve. Is there any response? Is the reflex mediated by one or both vagus nerves? How do you know? 14. Tighten the ligature around the left vagus near the cephalic end of the exposed portion and sever the nerve anterior to the ligature. Note any changes in respiration. Again stimulate the cephalic stump of the right depressor nerve and note the results. 15. Inject 0.20 ml of epinephrine. Is the effect on blood pressure stronger or weaker than before? Does it last longer? Why? 16. Inject 0.10 ml of 1/2,500 acetylcholine. Note effects on blood pressure and heart rate. Explain the results. 17. Examine the animal's pupils with good light. Inject 1 ml of 1% atropine sulfate via the IV drip. Do this a little at a time over 7-10 minutes. Look at the animal's pupils again. Are they maximally dilated? If not, consult the instructor. Stimulate the caudal end of the right vagus with twice the voltage that earlier produced marked inhibition of the heart. Is there any effect on blood pressure? 18. Inject 3 ml of 1/2,500 acetylcholine. How is the blood pressure affected? How do you explain this? What was the effect of the atropine? 19. Check with the instructor before injecting sodium pentobarbital to sacrifice the animal. ANALYSIS/PREPARING YOUR REPORT For each experimental manipulation, state the result you expected and why, the result you actually obtained, and if there is a difference, explain it. A diagram of the reflex circuits should be a help. Your report should include the relevant printouts of the data record with complete labels.