Gravimetric Analysis: Copper Sulphate Crystallisation
advertisement

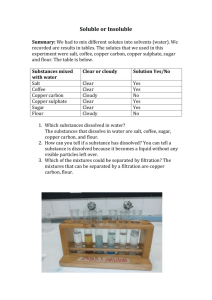
YEAR 12 PRACTICAL 5 – GRAVIMETRIC ANALYSIS Determining the water of crystallisation of copper (II) sulphate Copper sulphate exists in hydrated form, as CuSO4.xH2O. This form of copper (II) sulphate is blue. On heating, the hydrated copper sulphate loses its water to become anhydrous copper (II) sulphate. This process can be represented by the equation: CuSO4.xH2O(s) CuSO4(s) + xH2O(g) By weighing the copper sulphate sample before and after strong heating it is possible to calculate the value of x. Method 1. Weigh out accurately between 5 and 6 grams of hydrated copper sulphate, using a 2 decimal place balance. Weigh a crucible without any solid in it, add the correct amount of solid, and weigh the crucible again. 2. Place the crucible into a clay pipe triangle and heat with a roaring flame for five minutes. Then allow it to cool. When it is cool enough to touch weigh it again. 3. Place the crucible back in the gauze and heat strongly for a further three minutes. Then allow it to cool. When it is cool enough to touch weigh it again. 4. Repeat the process, heating for two minutes each time, until there is no further change in mass. Analysis First method: 1. Calculate the number of moles of anhydrous copper sulphate produced during the reaction. Hence deduce the number of moles of hydrated copper sulphate that was initially present. 2. Calculate the relative formula mass of hydrated copper sulphate and hence the value of x. Second method: 1. Calculate the mass of water lost during the experiment. 2. Calculate the moles of water lost and the moles of anhydrous copper sulphate produced. 3. Calculate the ratio of the moles of water to the moles of copper sulphate. This is the value of x. Evaluation 1. Deduce the overall percentage apparatus error, given that the percentage error in the mass balance is ±0.01 g and two critical measurements were made. 2. Using the first analytical method, work out the difference between your relative formula mass and the correct formula mass based on x = 5. 3. Hence calculate the percentage error in the experiment, and compare this to you the apparatus error. 4. Suggest two possible improvements, and explain why they would help.