SU-Energy_Efficiency_of_Different_Ways_of_HeatingWater_Take
advertisement

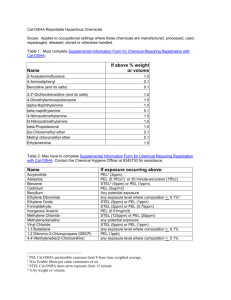
ENERGY AND THE ENVIRONMENT Comparing the Efficiency of Different Ways of Heating Water Take-Home Experiment Write-Up This purpose of this experiment was to investigate different ways of heating water at home, and to find the most efficient way. Three of the most common methods are heating water using a microwave, boiling it in a kettle or on a stove. To find the efficiency, water was heated in the three different ways and the temperature was recorded over a period of time using an oven thermometer. Using information about: a) the temperature change of the water over time b) the specific heat capacity of the water and c) the mass of the water, the total power used to heat the water was calculated, also measured using a power meter. . The total power input into the device was Finally, the efficiency was found as a ratio between the power used, and the power input, . More specifically, to determine the efficiency of the microwave, a 200mL glass of water was heated over different time intervals to measure the time dependency of the temperature. The heating time was set to 10s, increasing by 10s up to 150s (This is because, in order to read the thermometer, the microwave would have to be stopped and the door opened for each reading. Interrupting the heating in this way would cause inaccuracies. Instead, a fresh sample of water was used and the heating time was increased for each consecutive measurement) and both the initial temperature and final temperature were recorded. To minimize errors, the initial temperature was roughly uniform for all samples. To test the kettle, 1L of water was poured into it and heated up. The temperature was recorded for different time intervals, starting from 30s, increasing by 30s for each consecutive measurement, up to 300s. Finally, 2L of water was heated on a stove, and the temperature of the water was recorded every 10s up to 300s. In this case, "efficiency" means the power heating the water - a quantity we can calculate from temperature measurements - divided by the power consumed by the heating device - a quantity we can measure. For each experiment, a power meter was used to measure the power input to the heating device. Furthermore, before measuring the temperature of the water, the water was stirred to avoid temperature gradients in the fluid. The main formulas used are as follows: Efficiency , Puseul P el Pinput Pinput The power used, , is determined using the formula: Pel (c p )( m)( s ) Physics and Astronomy Outreach Program at the University of British Columbia Where is the specific heat capacity of the water, [J/K.g] , is the mass of the water that is heated [g] is the slope of temperature vs. time graph is measured using a power meter, a device that measures the power consumption of any electrical device. Physics and Astronomy Outreach Program at the University of British Columbia MICROWAVE The data obtained for the microwave is shown in Figure 1 below. The linear fit of the obtained data, yields a value of the slope . Using: a) Mass of the water used, b) The literature value of The electrical power put into the water is found to be: The power input, . as measured using the kilowatt meter, was 1031 W. The efficiency was thus found to be 40.6% Sample Calculations: Values 1. Electrical Power Used Pel (c p )( m)( s ) (4.18)( 200)(0.5) Pel 419W 2. Cp (J/K.g) 4.18 m (g) 200 s 0.50 Pel (W) 419 Average Pin(W) 1030.67 Efficiency Pel Pinput 419 0.406 41% 1031 Figure 1: Heating in a Microwave Physics and Astronomy Outreach Program at the University of British Columbia Physics and Astronomy Outreach Program at the University of British Columbia KETTLE The data obtained while heating 1L of water in a kettle is shown in Figure 2. The slope of the graph was determined to be: . Using: a) Mass of the water used, b) The literature value of The electrical power put into the water is found to be: The power input, . as measured using the kilowatt meter, was 1314 W. The efficiency was thus found to be 86% Sample Calculations: 3. Electrical Power Used Pel (c p )( m)( s ) (4.18)(1000)(0.27) Pel 1133 W 4. Values Cp (J/K.g) 4.18 m (g) 1000 s 0.27 Pel (W) 1133 Efficiency Pel Pinput 1133 0.86 86% 1314 Average Pin(W) Figure 2: Heating in a Kettle Physics and Astronomy Outreach Program at the University of British Columbia 1314 STOVE The data obtained while heating 2L of water on a stove top is shown in Figure 3. The slope of the graph was determined over the linear portion of the graph to be .. Notice how the graph slowly tapers off after t=2500 s to eventually become flat. This is where the water starts to boil and a phase change occurs; the heat being supplied to the water is used for evaporation and not to raise the temperature of the water. Using: c) Mass of the water used, d) The literature value of The electrical power put into the water is found to be: The power input, . as measured using the kilowatt meter, was 1240 W. The efficiency was thus found to be 20% Sample Calculations: 5. Electrical Power Used Pel (c p )( m)( s ) Values Cp (J/K.g) m (g) s 4.18 2000.0 0.03 Pel (W) 251 Average Pin(W) 1240.0 (4.18)( 2000)(0.03) Pel 251W 6. Efficiency Pel Pinput 251 0.20 20% 1240 Figure 3: Heating on a Stove Physics and Astronomy Outreach Program at the University of British Columbia Conclusion There is a noticeably large difference between the three methods of heating water. A kettle (efficiency = 86%) was found to be more than twice as efficient as a microwave (41% efficient) and 8 times as efficient as using a stove to heat water (even though the pot on the stove was covered to minimize heat loss to the surroundings). Anoushka Rajan 2010/12/19 Physics and Astronomy Outreach Program at the University of British Columbia