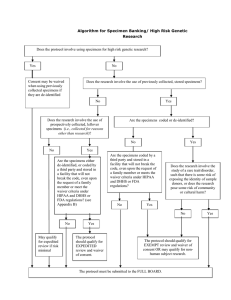
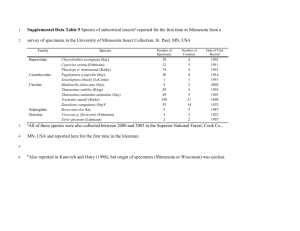
Specimen and Record Review
advertisement

Guideline for Writing a Protocol – Specimen and Record Review (RSRB Protocol Template Final v. 11/07/2014) NOTE: This template is to be used as a guideline for writing a protocol involving research use of subject specimens/records/data. For additional guidance regarding research that may qualify for expedited review versus an exempt determination, refer to Policy 501 Levels of RSRB Review. Title: Principal Investigator: 1. Purpose of the Study: What is your research topic and question? NOTE: Investigators conducting research in foreign countries should refer to the Guideline for Conducting International Research to ensure that items are addressed and additional protocol elements are considered and adequately described. 2. Background: What is the justification and rationale for the proposed study to support the purpose? If known, provide a summary of prior experience and/or history important to understand the proposed study. Include any relevant literature citations. 3. Study Population and Recruitment: How many subject specimens/records are needed to conduct the study? Is the number an appropriate size to achieve meaningful scientific and statistical results? Note: If a number of specimens/records will be reviewed with the expectation that you will collect/use data only from a sampling of them, state that here (e.g., We anticipate reviewing approximately X records/specimens. Of those records/specimens it is anticipated that approximately X subjects will meet the eligibility criterion.) How will subjects be identified (what is the source/location of records to be reviewed)? Does the study team have routine access to the data being reviewed? If not, how will the study team obtain the data? If a secondary analysis of pre-existing data, indicate the database to be used or where the data is coming from and how eligible subjects will be identified from the database. What is the gender and/or age range? What is the intended racial and ethnic distribution of the subjects? Provide justification for any enrollment restrictions based upon race or ethnic origin. What are the inclusion and exclusion criteria that define who will be eligible and who will not be eligible for the study (should support the purpose of the study)? 4. Informed Consent: How will consent be obtained (e.g., written or verbal consent, information letter)? Or, Provide justification for a waiver of consent or documentation of consent. Version Date: INSERT Page 1 of 2 5. Study Activities: Is this a retrospective or a prospective record/specimen review? o Retrospective means the data/specimens are already in existence when the study is submitted to the RSRB for initial review. o Prospective means the data/specimens don’t exist at the time the study is submitted to the RSRB for initial review. Indicate the period of time (duration) for when the data/specimens will be collected or were originally collected (mm/dd/yyyy to mm/dd/yyyy). If this is a retrospective review, the end date must be before the submission date to RSRB). If there is no end date, provide justification. What data points will be collected to meet the purpose of the study (include a copy of the data collection sheet in the RSRB application)? Will the Investigator or study team have access to any links or subject identifiers that will be recorded for purposes the study (i.e., will the data be collected or recorded in a de-identified manner)? Note: To determine whether you are recording identifiable information, refer to the URMC and Affiliates HIPAA Privacy Policy 0P30. To determine whether the study activities may qualify for exemption, refer to the Guideline for Determining Exempt Research. 6. Risks and Benefits: 6.1. Risks: What are the potential risks of conducting this study (e.g., confidentiality breach is a risk associated with chart review research)? 6.2. Benefits: What are the possible benefits from participation in the research? If there are no possible benefits, state no benefit. 7. Data Analysis: What are the statistical methods that will be used to analyze the data obtained during the study? 8. Data Storage and Confidentiality: How will data (both paper and electronic as applicable) be stored and who will have access to the data? How will the data be secured to maintain confidentiality of the data (i.e., coding data and using a secure data storage mechanism to prevent unauthorized access to the data)? If subject identifiers will be collected initially, specify if/when they will be removed from the data set and/or destroyed. NOTE: To ensure adequate encryption of files stored on portable devices such as laptops, tablets, memory sticks, etc., contact Information Technology (URMC website or UR website). For instruction regarding encryption of emails containing PHI see the Information Systems Email guidance. For information regarding maintaining subject privacy, the HIPAA website provides frequently asked questions, or contact the HIPAA privacy officer for assistance. 9. References: Provide complete, numbered references for all citations listed in the order that they appear. Version Date: INSERT Page 2 of 2