Master list of ionic compound solubility
advertisement
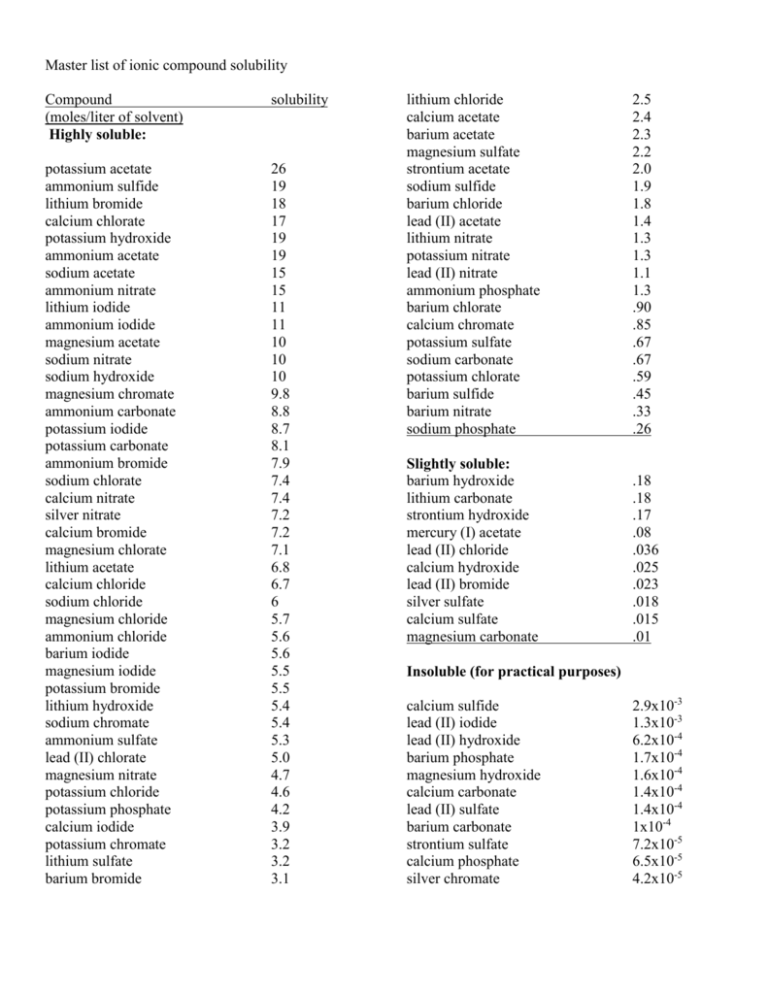
Master list of ionic compound solubility Compound (moles/liter of solvent) Highly soluble: solubility potassium acetate ammonium sulfide lithium bromide calcium chlorate potassium hydroxide ammonium acetate sodium acetate ammonium nitrate lithium iodide ammonium iodide magnesium acetate sodium nitrate sodium hydroxide magnesium chromate ammonium carbonate potassium iodide potassium carbonate ammonium bromide sodium chlorate calcium nitrate silver nitrate calcium bromide magnesium chlorate lithium acetate calcium chloride sodium chloride magnesium chloride ammonium chloride barium iodide magnesium iodide potassium bromide lithium hydroxide sodium chromate ammonium sulfate lead (II) chlorate magnesium nitrate potassium chloride potassium phosphate calcium iodide potassium chromate lithium sulfate barium bromide 26 19 18 17 19 19 15 15 11 11 10 10 10 9.8 8.8 8.7 8.1 7.9 7.4 7.4 7.2 7.2 7.1 6.8 6.7 6 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.0 4.7 4.6 4.2 3.9 3.2 3.2 3.1 lithium chloride calcium acetate barium acetate magnesium sulfate strontium acetate sodium sulfide barium chloride lead (II) acetate lithium nitrate potassium nitrate lead (II) nitrate ammonium phosphate barium chlorate calcium chromate potassium sulfate sodium carbonate potassium chlorate barium sulfide barium nitrate sodium phosphate 2.5 2.4 2.3 2.2 2.0 1.9 1.8 1.4 1.3 1.3 1.1 1.3 .90 .85 .67 .67 .59 .45 .33 .26 Slightly soluble: barium hydroxide lithium carbonate strontium hydroxide mercury (I) acetate lead (II) chloride calcium hydroxide lead (II) bromide silver sulfate calcium sulfate magnesium carbonate .18 .18 .17 .08 .036 .025 .023 .018 .015 .01 Insoluble (for practical purposes) calcium sulfide lead (II) iodide lead (II) hydroxide barium phosphate magnesium hydroxide calcium carbonate lead (II) sulfate barium carbonate strontium sulfate calcium phosphate silver chromate 2.9x10-3 1.3x10-3 6.2x10-4 1.7x10-4 1.6x10-4 1.4x10-4 1.4x10-4 1x10-4 7.2x10-5 6.5x10-5 4.2x10-5 lead (II) sulfide silver phosphate barium chromate silver carbonate barium sulfate magnesium phosphate silver chloride mercury (I) chloride lead (II) carbonate lead (II) chromate lead (II) phosphate mercury (I) bromide 3.6x10-5 1.6x10-5 1.3x10-5 1.2x10-5 9.4x10-6 7.6x10-6 6.2x10-6 6.25x10-6 4.1x10-6 1.8x10-7 1.7x10-7 2.4x10-8 The borderlines of these groupings is not defined. This is just my opinion. Ammonium Barium Calcium Lead (II) Lithium Magnesium Mercury (I) Potassium Silver Sodium Strontium List patterns that you see (including their exceptions): sulfide sulfate phosphate nitrate iodide hydroxide = insoluble (for practical purposes!) chromate chloride chlorate = slightly soluble carbonate bromide acetate = soluble Alkali metals and ammonium salts, Whatever they may be, Can always be depended on for solubility Every single sulfate Is soluble, 'Tis said 'Cept barium, strontium, mercury one And calcium and lead. When asked about the nitrates The answer is always clear, They each and all are soluble, Is all we want to hear. Hydroxides in general don't dissolve at all But, barium, strontium and calcium Are slightly soluble Most every chloride's soluble At least we've always read Save silver, mercury one And chloride of lead The carbonates are insoluble, It's lucky that it is so, Or else, our marble buildings Would melt away like snow. Alkali metals and ammonium salts Whatever they may be Can always be depended on For solubility Alkali metals and ammonium salts Whatever they may be Can always be depended on For solubility SOLUBILITY GUIDELINES (for Ionic Reactions) Soluble Compounds NOT precipitates Nitrates Acetates Chlorates Chlorides Bromides Iodides Sulfates Exceptions PRECIPITATES None None None Ag+1, Hg2+2, Pb+2 Ag+1, Hg2+2, Pb+2 Ag+1, Hg2+2, Pb+2 Ca+2, Sr+2, Ba+2, Hg2+2, Pb+2 Insoluble Compounds PRECIPITATES Sulfides Carbonates Phosphates Hydroxides Chromates Exceptions NOT Precipitates NH4+1, Li+1, Na+1, K+1, Ca+2, Sr+2, Ba+2 NH4+1, Li+1, Na+1, K+1 NH4+1, Li+1, Na+1, K+1 Li+1, Na+1, K+1, Ca+2, Sr+2, Ba+2 NH4+1, Li+1, Na+1, K+1, Ca+2, Mg+2






