About 1 x 106 cells were lysed in 75oC warm lysis buffer (1
advertisement

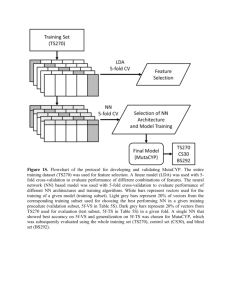
Supplementary Data Perinuclear positioning of the inactive human cystic fibrosis gene depends on CTCF, A-type lamins and an active histone deacetylase Joscha S. Muck1#, Karthikeyan Kandasamy1#, Andreas Englmann2,3, Martin Günther1 and Daniele Zink1* 1 Institute of Bioengineering and Nanotechnology (IBN), Department of Cell and Tissue Engineering, 31 Biopolis Way, The Nanos, Singapore 138669 2 Ludwig-Maximilians-Universität (LMU) München, Biozentrum, Department Biologie II, Grosshadernerstr. 2, 82152 Planegg-Martinsried, Germany 3 Current Address: Sabel Realschule München, Schwanthalerstraße 51-57, 80336 München, Germany # Both first authors contributed equally to the work * Corresponding author. Telephone: +65 6824-7107, fax: +65 6478 9080, e-mail: dzink@ibn.a-star.edu.sg 1 Supplementary Figure Legends Figure S1: Controls for experiments addressing gene positioning. Gene positioning was determined by FISH and 2D erosion analysis. Nuclei were subdivided into five concentric shells. For each condition, the five bars indicate the percentages of genespecific FISH signals in the different shells (mean +/- SEM; light grey: peripheral shell, black: center). At least three replicates were analyzed in each case and the numbers above the diagrams indicate the numbers of FISH signals evaluated. Asterisks denote significant (p < 0.05) changes in the numbers of FISH signals in the outermost shell (compared to control cells). a) The positioning of CFTR was determined in primary human renal proximal tubular epithelial cells (HPTC). HPTC were treated for 20 minutes (min) or for 10 hours (h) with TSA (control: untreated). b) The positioning of CFTR was determined in HeLa cells. HeLa cells were treated with CTCF-specific siRNA or with unspecific siRNA or were left untreated (control). c) The positioning of the -globin locus was determined in untreated cells (control) and in cells that were either treated for 10 hours with TSA or that were treated with CTCF-specific siRNA. Neither treatment led to significant changes in the positioning of the -globin locus. Figure S2: The positioning of CFTR was determined in SH-EP N14 and HEK 293 cells by FISH and 2D (left-hand panels) or 3D (right hand panels) erosion analysis. The bars show the mean +/- standard deviation. For further explanations of diagrams see legend of figure 2. In the 3D erosion analyses no signals were detected in the central 1-2 zones and 2 therefore only 3-4 bars are displayed in the diagrams. Asterisks denote significant (p < 0.05) changes in the numbers of FISH signals in the outermost shell. Figure S3: Comparison of our ChIP-chip data with results from the ENCODE project. a) The region covered by the DNA microarray used here is displayed and the genomic positions on chromosome 7 (Mb, top) as well as the gene loci (RefSeq, bottom, vertical lines mark exons) are indicated. The figure shows the enrichment profiles (scale on yaxis) obtained in our study by ChIP-chip for H3ac and H4ac with untreated HeLa cells (black; identical with the black profiles in Fig. 3). The enrichment profiles of H3ac and H4ac obtained by the ENCODE project with untreated HeLa cells are shown in blue (the ENCODE project and our study used HeLa S3 cells obtained from ATCC). Transcription factor binding sites (TF) mapped by the ENCODE project by using 23 different cell lines and addressing 60 different transcription factors are shown below the profiles. The numbers of binding sites mapped at a given site are indicated by the grey value of the vertical line (the same data set is displayed in a different way in panel b). In addition, DHS either mapped by the ENCODE project (DHS1) or by Ann Harris and co-workers (DHS2) (Mouchel et al. 2004, McCarthy & Harris 2005, Blackledge et al. 2007, Blackledge et al. 2009, Ott et al. 2009) are shown. In both cases multiple cell lines were used to map DHS and any particular cell line only displays a subset of the DHS compiled here. b) The region covered by the DNA microarray is displayed and the gene loci (RefSeq, bottom, vertical lines mark exons) are indicated. The H3ac profiles of TSAtreated HeLa cells (black; identical with the pink profile displayed in lane 1 of Fig. 3) and of Calu-3 cells (red; identical with the profile displayed in lane 2 of Fig. 3) are shown 3 (overlaid). Below the H3ac profiles all of the transcription factor binding sites identified by he ENCODE project by using 23 cell lines and investigating 60 different transcription factors are displayed. Each mapped binding site is symbolized by a grey square. Regions A-E are highlighted in yellow. Figure S4: Validation of the H4ac ChIP-chip data by ChIP-qPCR. For explanation of the diagrams see legend of figure 5. The results obtained from untreated control cells are shown in light grey and the data derived from TSA-treated cells are displayed in dark grey. No sites could be identified that are consistently more highly enriched in H4ac when CFTR localizes in the nuclear interior. Figure S5: Enrichment of H3K4me3, H3K9me3 and H3K27me3 determined by ChIPchip. a) Map of the region covered by the DNA microarray. The genomic positions on human chromosome 7 are indicated at the top (Mb) and the gene loci are displayed at the bottom (grey). Black arrows indicate the direction of transcription. b-d) HeLa cells were left untreated (control, red) or were treated for 20 minutes (blue), 40 minutes (green) or 60 minutes (brown) with TSA. ChIP was performed with an antibody against H3K4me3 (b), H3K9me3 (c) or H3K27me3 (d). The graphs display the enrichment profiles of the different histone H3 modifications (relative to histone H3, log2). The x-axis displays the positions on chromosome 7. Figure S6: Validation of ChIP-chip data obtained for H3K4me3, H3K9me3 and H3K27me27 by qPCR. a) Specific primer pairs were generated for those sites within 4 CFTR and adjacent regions that appeared to display some changes in the patterns of histone H3 methylation after 20 minutes of TSA treatment when histone H3 methylation patterns were determined by ChIP-chip (see Supplementary Data, Fig. S3). These primer pairs are labeled 1a – 16a. In addition, primers selected from the set of 63 primers pairs (Fig. 5) specific for CTCF binding sites and other sites were included (not labeled with a; CTCF sites are marked by arrows at the bottom of panel d). The positions of all primer pairs are shown in panel a (genomic positions on chromosme 7 are displayed at the top; gene loci are shown as grey boxes). Panels b-d) show the enrichment of H3K4me3 (b), H3K9me3 (c) and H3K27me3 (d) relative to histone H3 (scale on y-axis, the x-axis displays the primer positions). The bars (mean +/- SEM) show the values obtained with untreated control HeLa cells (light grey) and with HeLa cells treated for 20 minutes (min) with TSA (dark grey). A significant (p < 0.05) change was observed only in case of H3K9me3 at one single site (asterisk). Figure S7: Effects of TSA treatment on CTCF binding. Specific primer pairs were generated for CTCF binding sites mapped by the ENCODE project (1c – 7c; negative controls: N1 – N3). Primer pairs 29, 30, 50 and 54 were derived from the set of 63 primer pairs described above (figure 5). The positions of the primer pairs on chromosome 7 (Mb scale on top) and relative to the genes (grey, direction of transcription indicated by black arrowheads) are shown in panel a). Relative CTCF enrichment (ChIP/input; scale on yaxis in panels b and c) was determined by ChIP-qPCR using untreated Calu-3 cells (control, black bars) as well as HeLa (panel b) and HEK 293 (panel c) cells. HeLa and HEK 293 cells were either left untreated (control, white bars) or were treated for 20 min 5 (light grey bars) or 10 hours (dark grey bars) with TSA. The bars show the mean +/SEM. Figure S8: Panel (a) shows an immunoblot with lysates from HeLa cells. The cells were either untreated (control: c) or treated with lamin A/C-specific siRNA (siRNA). For detection antibodies against lamin A/C and -actin (loading control) were used. The positions of the bands of the size marker and the sizes of the respective peptides (kDa) are indicated on the left. The images in (b) show HeLa cells after immunofluorescent staining with an anti-lamin A/C antibody. Untreated control cells (left-hand panels) or cells treated with lamin A/C-specific siRNA (right-hand panels) were used. DAPI (DNA) staining is shown at the top and in blue in the merges at the bottom. The lamin A/Cspecific immunofluorescence is shown in the middle and in red at the bottom. Scale bars: 50 m. Figure S9: The positioning of CFTR was determined in HeLa cells by FISH and 2D (left-hand panel) or 3D (right hand panel) erosion analysis. Cells were untreated (control) or treated with lamin A/C-specific siRNA. The bars show the mean +/- standard deviation. For further explanations of diagrams see legend of figure 2. In the 3D erosion analyses no signals were detected in the central 1-2 zones and therefore only 3-4 bars are displayed in the diagrams. Asterisks denote significant (p < 0.05) changes in the numbers of FISH signals in the outermost shell. 6 Figure S10: Acetylation of CTCF. The experiments were performed with HeLa cells which were either left untreated (control) or were treated for 20 minutes or for 10 hours with TSA. In addition untreated (control) Calu-3 cells were used. The four left-hand lanes show the results obtained by immunoblotting when cell lysates were used. The immunoblots (IB) were probed with antibodies against CTCF, H3ac, H4ac and -actin (loading control). The sizes (kDa) and positions of the size marker bands are indicated on the left. Immunoblotting was also performed after immunoprecipitation (IP) with an antibody specific for acetylated proteins (right-hand lanes). As a control IP with a nonspecific antibody (NS-IgG) was performed. 7 References Blackledge NP, Carter EJ, Evans JR, Lawson V, Rowntree RK, Harris A (2007) CTCF mediates insulator function at the CFTR locus. Biochem J 408: 267-275. Blackledge NP, Ott CJ, Gillen AE, Harris A (2009) An insulator element 3' to the CFTR gene binds CTCF and reveals an active chromatin hub in primary cells. Nucleic Acids Res 37: 1086-1094. McCarthy VA, Harris A (2005) The CFTR gene and regulation of its expression. Pediatr Pulmonol 40: 1-8. Mouchel N, Henstra SA, McCarthy VA, Williams SH, Phylactides M, Harris A (2004) HNF1alpha is involved in tissue-specific regulation of CFTR gene expression. Biochem J 378: 909-918. Ott CJ, Blackledge NP, Kerschner JL et al. (2009) Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc Natl Acad Sci U S A 106: 19934-19939. 8