Spectroscopic Study of the Simplest Criegee Intermediate
advertisement

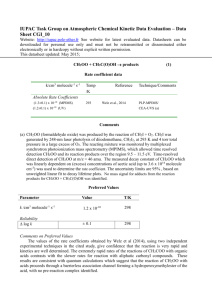
Spectroscopic Study of the Simplest Criegee Intermediate David Feng Mentor: Craig Murray Criegee intermediates (CIs) are formed in the ozonolysis of alkenes, a major removal mechanism for unsaturated hydrocarbons in the troposphere. CIs are recognized as key species in tropospheric formation of secondary organic aerosols and are thought to play a role in atmospheric regulation of OH. We use cavity ring-down spectroscopy to identify and study the simplest CI, formaldehyde oxide (CH2OO , through the B̃1A′–X̃1A′ absorption band. CH2OO is produced by the reaction of iodomethyl radical (CH2I) with molecular oxygen; CH2I itself is formed from the photolysis of diiodomethane (CH2I2) at 355-nm. We will report highresolution spectra of vibronic bands in the wavelength range 415 – 430 nm. The absence of resolvable rotational structure in these bands suggests that the B̃1A′ state is strongly dissociated, even at low excitation energies. We will address the discrepancies between experimental measurements of this absorption band.