La tuberculosis y VIH - Grupo de Mujeres Argentinas
advertisement

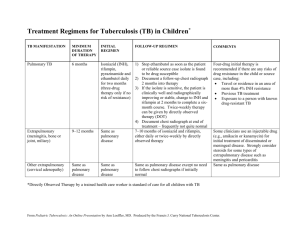
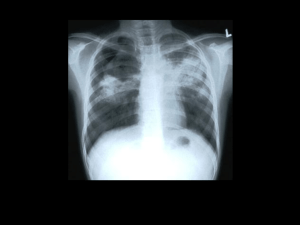
La tuberculosis y VIH VIH el InSite Base de conocimiento Capítulo El 2003 de mayo Lisa Goozé, MD, la Universidad de Stanford, Charles L. Daley, MD, la Universidad de California, San Francisco, Lisa Goozé, MD, la Universidad de Stanford, Charles L. Daley, MD, la Universidad de California, San Francisco, La introducción La epidemiología de Tuberculosis VIH-relacionada La Tuberculosis droga-resistente El impacto de Infección de VIH en la Patogénesis de Tuberculosis El impacto de Tuberculosis en la Historia Natural de Infección de VIH La Presentación clínica y Diagnóstico La Presentación clínica de Tuberculosis Los Resultados de Radiographic Los Exámenes bacteriológicos El tratamiento de Tuberculosis VIH-relacionada Rifampin - vs. los Régimenes Rifabutin-basados La seguridad y Tolerability Las Society/CDC Tratamiento Recomendaciones Torácicas americanas actuales Las Interacciones del droga-droga La Reacción paradójica La iniciación de Terapia de Antiretroviral en el Paciente de Coinfected El diagnóstico y Tratamiento de Infección de la Tuberculosis Latente El diagnóstico de Infección de la Tuberculosis Latente El tratamiento de Infección de la Tuberculosis Latente Las Recomendaciones de Society/CDC Torácicas americanas actuales Las referencias Las Mesas Mesa 1: La incidencia Tasa de Tuberculosis Entre las Cohortes de VIH-seropositivo y - los Individuos Seronegativos Mesa 2: Los resultados de Terapia en los Estudios Probables de 6-mes Tuberculosis Tratamiento Régimenes Mesa 3: Los Régimenes de Droga recomendados para el Tratamiento de Tuberculosis Droga-susceptible Mesa 4: El primero-línea Agentes de Antituberculosis Mesa 5: Los Inhibidores de Protease y Rifamicinas Mesa 6: Nonnucleoside Reverse los Inhibidores de Transcriptase y Rifamicinas Mesa 7: Los Estudios Aleatorizados probables de Tratamiento de Infección de la Tuberculosis Latente en los Pacientes Infectados con HIV Mesa 8: Los régimenes para el Tratamiento de Infección de la Tuberculosis Latente Las figuras Figure 1: Las TB/HIV Coinfection Proporciones estimadas, 1997, Figure 2: La Radiografía del pecho de un Paciente Infectado con HIV con la Tuberculosis Diseminada Figure 3: La Radiografía del pecho de un Paciente Infectado con HIV con la Tuberculosis Los Recursos relacionados Las actualizaciones Los Capítulos de la Base de conocimiento relacionados Otros Capítulos de Books/Book Las pautas Los Informes Los Artículos La Educación continua Las imágenes Los eslabones La introducción La tuberculosis y VIH han sido estrechamente vinculados desde la emergencia de AYUDAS. La infección de VIH ha contribuido a un aumento significante en la incidencia mundial de tuberculosis. (1,2) produciendo un declive progresivo en la inmunidad célulamediada, VIH altera la patogénesis de tuberculosis, mientras aumentando el riesgo de desarrollar la enfermedad en los individuos del coinfected y llevar al envolvimiento del extrapulmonary más frecuente y las manifestaciones del radiographic atípicas grandemente. Aunque la tuberculosis VIH-relacionada es el treatable y evitable, las proporciones de incidencia continúan subiendo en naciones en vías de desarrollo dónde la infección de VIH y tuberculosis son endémicas y los recursos están limitados. Mundial, la tuberculosis es la infección oportuna más común que afecta el individuals,(1 VIH-seropositivo) y es la causa más común de muerte en los pacientes con las AYUDAS. (3) Este capítulo repasará la epidemiología, patogénesis, y dirección de tuberculosis en la escena de infección de VIH. La epidemiología de Tuberculosis VIH-relacionada La Organización Mundial de la Salud (QUIÉN) estima que se infectan un tercio de la población del mundo con la tuberculosis de Mycobacterium, mientras resultando en un estimó 8 millones de nuevos casos de tuberculosis y casi 2 millones de muertes cada año. (4) se estiman Aproximadamente 10 millones de personas para ser el coinfected con la tuberculosis de M y VIH, y encima de 90% de estos individuos dualmente infectados resida en las naciones en vías de desarrollo. En algunas áreas de Africa subsahariana, las proporciones de coinfection exceden 1,000 por 100,000 población (Figura 1). (5) Mundial, la tuberculosis es la causa más común de muerte entre los pacientes con las AYUDAS, matando 1 de cada 3 pacientes. (3) Después de décadas de declive firme, los casos de la tuberculosis aumentaron en 1986 en los Estados Unidos. (6) Entre 1985 y 1990, casos de la tuberculosis aumentados por 20%, mientras produciendo 28,040 exceso embala de tuberculosis. Los Centros americanos para el Mando de la Enfermedad y Prevención (CDC) estima que la tuberculosis SIDArelacionada respondió de un mínimo de 30% de éstos los casos del exceso. (7) Afortunadamente, los casos de la tuberculosis han estado rechazando en los Estados Unidos desde 1992.(8) Entre 1992 y 1999, casos de la tuberculosis disminuidos por casi 34%. los casos VIH-relacionados también han rechazado. De 1993 a 1998, la proporción de casos VIH-relacionados ha disminuido de 29% a 20% en los 25 - al grupo etario 44-año-viejo. (8) El predominio de infección de VIH entre los casos de la tuberculosis varía grandemente del estado para declarar. Desgraciadamente, en 1999, sólo medio los estados hicieron a VIH probar los resultados en >=75% de casos de la tuberculosis. (8) Para estas jurisdicciones, la VIH seroprevalence proporción mala era 17.9%, con un rango de 0-44.1%. El Distrito de Columbia, Florida, y Ciudad de Nueva York que cada uno tenía el seroprevalence de VIH tasa >40% entre sus casos de la tuberculosis, considerando que Texas, Carolina del Sur, Utah, y Georgia tenían las proporciones >=30%. El declive en la tuberculosis VIH-relacionada en los Estados Unidos y otros países industrializados han parangonado un declive global en los casos de la tuberculosis. Si o no el uso de terapia del antiretroviral eficaz (el ARTE) ha acelerado este declive no está claro. Dos estudios de la cohorte han descrito la frecuencia de tuberculosis durante la era actual de tratamiento. (9,10) En el Francfort SIDA cohorte estudio de 1,000 hombres homosexuales Infectados con HIV con el T-linfocito de CD4 cuenta <200 cells/mm3, la incidencia global de SIDA-definir las condiciones disminuida por >70% entre 1992 y 1996.(9) Sin embargo, la proporción de tuberculosis, aunque bajo, permanecido estable encima del período del estudio. En el contraste, el EuroSIDA cohorte estudio de 7,000 pacientes Infectados con HIV informó los declives dramáticos en la proporción de tuberculosis y diseminó el avium de Mycobacterium complejo (MAC) del período antes de 1993 al período después de las 1997, coincidiendo con la introducción de ARTE potente. (10) se necesitan los estudios Extensos evaluar el impacto de terapia del antiretroviral en las proporciones de tuberculosis en las poblaciones diferentes. Drug-Resistant Tuberculosis Paralleling the increase in tuberculosis cases in the United States was an increase in the number of cases of drug-resistant tuberculosis. The frequency of multidrug-resistant tuberculosis (MDRTB) in the United States increased from 0.4% in the early 1980s to 3.5% in 1991, with most of the MDRTB cases residing in New York.(11) The CDC investigated at least eight MDRTB outbreaks that occurred in New York, New Jersey, and Miami, and reported that approximately 90% of the cases were HIV seropositive.(12) In New York, previously treated patients, those with HIV infection, and injection drug users were all at increased risk of having drug-resistant tuberculosis.(13) A study of HIV-infected and uninfected individuals with tuberculosis in eight U.S. centers participating in a communitybased clinical trials group found a 20.4% prevalence of tuberculosis resistant to one or more drugs, with 5.6% of the isolates resistant to both isoniazid (INH) and rifampin.(14) In multiple logistic regression analyses, HIV infection was shown to be a risk factor for having drug-resistant tuberculosis, independent of geographic location, history of prior therapy, age, or race. Recent studies have found that HIV-seropositive patients are more likely to develop acquired drug resistance (ADR) than seronegative cases.(15-18) In a case-control study involving 16 cases of ADR in San Francisco between 1990 and 1994, AIDS, nonadherence to the tuberculosis treatment regimen, and gastrointestinal symptoms were each independently associated with the acquisition of drug resistance.(17) During the study period one of every 16 AIDS patients with tuberculosis, and either gastrointestinal symptoms or nonadherence, developed ADR. Of note, most of the patients developed mono-rifampin-resistant tuberculosis, which is an unusual form of ADR. It is not clear why acquired mono-rifampinresistant tuberculosis would be more likely to arise in HIV-1-seropositive persons, although malabsorption of antituberculosis drugs has been postulated as a causative factor.(17) Impact of HIV Infection on the Pathogenesis of Tuberculosis Tuberculosis can develop through progression of recently acquired infection (primary disease), reactivation of latent infection, or exogenous reinfection. Recent data suggest that in urban areas within the United States, recent transmission accounts for a larger proportion of cases than was realized previously.(19,20) Molecular genotyping studies in San Francisco and New York reported that 30-40% of new cases were due to recent infection with rapid progression to disease. In both studies, HIV infection or AIDS was an independent risk factor for recent acquisition of infection and rapid progression to disease. Infection with M tuberculosis can occur when an individual exposed to an infectious case of tuberculosis inhales particles (<5 µm in size) containing the tubercle bacilli.(21) If the bacilli reach the pulmonary alveoli, they may be ingested by alveolar macrophages, the first line of defense against M tuberculosis. Surviving tubercle bacilli multiply within the macrophage and eventually undergo hematogenous spread to other areas of the body. Although defects in macrophage function have been demonstrated in HIV-infected patients, there is no conclusive evidence that HIV-seropositive persons are more likely to acquire tuberculous infection than HIV-seronegative individuals, given the same degree of exposure.(22) Once infection does occur, however, the risk of rapid progression is much greater among persons with HIV infection, because HIV impairs the host's ability to contain new tuberculous infection. Immunocompetent individuals infected with M tuberculosis have approximately a 10% lifetime risk of developing tuberculosis,(23) with half of the risk occurring in the first 1-2 years after infection. In contrast, a San Francisco study reported rapid spread of tuberculosis during an outbreak in a residential care facility for HIVseropositive substance abusers.(24) Among the 30 residents who were exposed to possible infection, 11 (37%) developed tuberculosis within 106 days, and at least one person developed tuberculosis within 4 weeks of exposure, demonstrating the rapidity with which tuberculosis can spread within an HIV-infected population. Several cohort studies have demonstrated a high incidence of active tuberculosis among HIV-infected individuals with positive tuberculin skin test results (Table 1).(25-30) However, the rates of active tuberculosis have varied considerably depending on the population and region studied. For example, among cohorts of HIV-infected injection drug users with positive skin tests, the annual rate of tuberculosis has varied from 4.5 to 10.4 cases per 100 person-years. These variations in the incidence rate of tuberculosis are likely due to differences in the prevalence of actual tuberculous infection (as opposed to false-positive skin tests) among the cohorts, differences in the severity of immunosuppression, and the amount of ongoing transmission that was occurring in the cohorts. Infection with M tuberculosis in an immunocompetent person is thought to confer significant protective immunity against exogenous reinfection.(23) However, reinfection has been reported in HIV-seronegative(31,32) and -seropositive individuals,(33-37) although its incidence is not known. DNA fingerprinting on paired isolates of M tuberculosis from 17 patients who had repeatedly positive cultures at a single hospital in New York City found four patients to have acquired a new, drug-resistant strain of M tuberculosis through exogenous reinfection, probably as a result of nosocomial transmission.(33) Because of the increased virulence in immunocompetent hosts of M tuberculosis compared with other opportunistic pathogens (eg, Pneumocystis jiroveci), tuberculosis can occur early in the course of HIV infection. In several studies of HIV-infected patients with pulmonary tuberculosis, the median CD4 T-cell count was >300 cells/mm3.(38) However, in patients with primarily extrapulmonary involvement or disseminated disease, the CD4 T-cell count may be much lower. For example, two studies in African patients with disseminated disease found the median CD4 T-cell count to be <80 cells/mm3.(38) A prospective study in the United States,(25) found the median CD4 T-cell count to be 144 cells/mm3 (range 2-543) in HIV-infected patients with all forms of tuberculosis. Although tuberculosis can be a relatively early manifestation of HIV-1 infection, it is important to note that the risk of developing tuberculosis, and of disseminated infection, increases as the CD4 T-cell count decreases. Impact of Tuberculosis on the Natural History of HIV Infection Although the immune response to M tuberculosis is important in controlling disease, immune activation may also be associated with increased HIV viral load and accelerated progression of HIV infection. A retrospective cohort study in the United States found that although only one patient in the group died of tuberculosis, HIV-infected patients with tuberculosis do not survive as long as HIV-infected controls without tuberculosis, even after controlling for baseline CD4 T-cell count. When tuberculin-positive HIV-infected patients were given INH therapy in Haiti, they were less likely to develop AIDS and less likely to die than patients given placebo.(39) Thus, it is likely that tuberculosis acts to accelerate the clinical course of HIV infection. Although increased viral replication is thought to play a role, the mechanisms by which tuberculosis accelerates progression of HIV disease are not known with certainty. High levels of tumor necrosis factor (TNF)-alpha, which are known to increase HIV replication in T-cell clones,(40) have been demonstrated in both HIV-1-seropositive and -seronegative tuberculosis cases.(41). Moreover, investigators have shown that M tuberculosis or purified protein derivative can also increase viral replication in infected T lymphocytes and monocytes.(42-44) A recent study demonstrated a 5- to 160-fold increase in viral replication during the acute phase of untreated tuberculosis.(40) The clinical significance of this increase in viral load is uncertain. Clinical Presentation and Diagnosis Clinical Presentation of Tuberculosis The clinical presentation of pulmonary tuberculosis can vary widely in both immunocompetent and immunocompromised hosts. In general, the presentation in the HIVinfected patient is similar to that seen in HIV-uninfected patients, although the signs and symptoms (such as fevers, weight loss, and malaise) may be attributed to HIV itself and the possibility of tuberculosis overlooked. Symptoms are usually present for weeks to months, and the acute onset of fever and cough is more suggestive of a nonmycobacterial pulmonary process. If there is no response to antimicrobial therapy, however, the possibility of tuberculosis should be considered. In HIV-infected patients, clinical manifestations of pulmonary tuberculosis reflect different levels of immunosuppression. Earlier in the course of HIV disease, tuberculosis is more likely to present as classical reactivation-type disease, whereas patients with advanced immunosuppression are more likely to present with findings consistent with primary tuberculosis (see Radiographic Findings, below). The prevalence of extrapulmonary tuberculosis is increased in HIV-infected patients. Low CD4 T-cell counts are associated with an increased frequency of extrapulmonary tuberculosis, positive mycobacterial blood cultures, and atypical chest radiographic findings, reflecting an inability of the impaired immune response to contain infection.(45) Patients with extrapulmonary tuberculosis may present with signs and symptoms specific to the involved site, such as lymphadenopathy, headache, meningismus, pyuria, abscess formation, back pain, or abdominal pain. These findings in HIV-infected patients can present a diagnostic challenge. Whenever possible, diagnostic specimens should be examined for acidfast bacilli (AFB) and cultured for mycobacteria. Radiographic Findings The chest radiograph is the cornerstone of diagnosis for pulmonary tuberculosis. Upper lobe infiltrates and cavities are the typical findings in reactivation tuberculosis, whereas intrathoracic lymphadenopathy and lower lobe disease are seen in primary tuberculosis. In HIV-infected persons with higher CD4 T-cell counts (eg, >200 cells/mm3) the radiographic pattern tends to be one of reactivation disease with upper lobe infiltrates with or without cavities.(46) In HIV-infected persons who have a greater degree of immunosuppression (eg, CD4 T-cell count <200 cells/mm3), a pattern of primary disease with intrathoracic lymphadenopathy and lower lobe infiltrates is seen (Figure 2 and Figure 3). As chest radiographs may appear normal in 7-14% of cases, a high index of suspicion must be maintained in evaluating an HIV-infected patient with symptoms suggestive of tuberculosis.(47,48) In the authors' experience, the finding of low-density lymph nodes with peripheral enhancement on a contrast-enhanced chest computed tomography (CT) scan is highly predictive of tuberculosis. Bacteriologic Examinations All patients suspected of having pulmonary tuberculosis should have 3 sputum specimens obtained on 3 consecutive days, and these specimens should be examined for AFB and cultured for mycobacteria. AFB identified on smear are not diagnostic of tuberculosis, as the acid-fast stain detects mycobacteria other than M tuberculosis, including M aviumintracellulare complex or M kansasii. However, until identification is confirmed, empiric therapy for tuberculosis should be initiated if the sputum smear is positive for AFB. The rate of AFB smear positivity has varied from 31% to 89% in HIV-positive patients.(49) In general, the rate of smear positivity correlates with the extent of radiographic disease. For example, patients with cavitary lesions due to active tuberculosis will almost always have positive smears, whereas a negative smear in a patient with minimal disease on chest radiograph would not be unusual, and would not rule out active tuberculosis. However, in HIV-infected patients positive smears may be seen with relatively little radiographic involvement. When expectorated sputum specimens are AFB smear-negative, further evaluation may be indicated. Bronchoscopy with bronchoalveolar lavage and transbronchial biopsy may be useful in the evaluation of an abnormal chest radiograph when sputum smears are negative. In this setting, a rapid diagnosis of presumptive tuberculosis, based on histology and AFB smear of specimens obtained by bronchoscopy, can be made in 30-40% of individuals, which is similar to the yield of bronchoscopy in HIV-negative cases with smear-negative pulmonary tuberculosis.(50). Positive cultures for M tuberculosis provide a definitive diagnosis of tuberculosis. Approximately 15% of reported tuberculosis cases are culture negative, but these data are not available for HIV-infected cases. However, at San Francisco General Hospital, culturenegative tuberculosis in HIV-infected patients is seldom observed. (This perceived increase in sensitivity may be due in part to the increased use of diagnostic bronchoscopy in HIVpositive cases of suspected tuberculosis.) Unfortunately, culture results may not be available for 2-6 weeks, creating a need for more rapid diagnostic techniques. Nucleic acid amplification (NAA) tests detect nucleic acid sequences unique to organisms in the M tuberculosis complex, allowing for a rapid diagnosis. Two NAA tests, the Amplified Mycobacterium Tuberculosis Direct Test (MTD; Gen-Probe) and the Amplicor Mycobacterium Tuberculosis Test (Amplicor; Roche) have been approved by the U.S. Food and Drug Administration (FDA) for use in respiratory specimens in patients who have not previously been treated for tuberculosis. The MTD test is approved for use in smear-positive or smear-negative samples, whereas Amplicor is only approved for use with smear-positive samples. A suggested algorithm and guide for interpreting NAA test results has recently been published,(51) but local tuberculosis control agencies may have their own guidelines for the use of these newer tests. A negative NAA test does not rule out the diagnosis of active tuberculosis, and antituberculous therapy and further diagnostic workup are needed if sufficient clinical suspicion for tuberculosis exists. The predictive value of NAA testing will vary depending on the sensitivity and specificity of the test in the local laboratory, as well as on the prevalence of M tuberculosis and other mycobacteria. Moreover, NAA testing does not provide information on drug resistance. NAA tests are an important addition to our armamentarium of diagnostic tools, but they do not replace AFB smear, culture, or, more importantly, clinical judgment. Treatment of HIV-Related Tuberculosis HIV-seropositive patients with tuberculosis respond well to antituberculosis therapy, as long as the regimen contains INH and a rifamycin. The treatment of HIV-related tuberculosis requires close monitoring because of frequent drug toxicities, possible drug-drug interactions, and paradoxical reactions. There have been six prospective studies, four randomized and two observational in nature, of 6-month treatment regimens for active tuberculosis (Table 2).(52-57) Because these studies differed in design, patient population, eligibility criteria, site of disease, frequency of dosing, dose administration, and outcome definitions, it is difficult to provide meaningful cross-study comparisons. It is important to note, however, that all of the studies reported a good, early clinical response to therapy, and the time in which M tuberculosis sputum cultures converted from positive to negative and treatment failure rates were similar to those in patients without HIV infection. The recurrence rates vary between studies. Although most studies reported recurrence rates of 5% or less among HIV-infected cases, two studies reported rates close to 10%. In Kinshasa, Zaire, patients with tuberculosis were treated with INH, rifampin, pyrazinamide, and ethambutol daily for 2 months, followed by INH and rifampin twice weekly for 4 months.(52) Only one-half of the doses in the continuation phase were directly observed. The HIV-seropositive cases were then randomized to receive an additional 6 months of placebo or twice-weekly INH and rifampin. There was no statistically significant difference in the recurrence rates between the HIV-seropositive (9%) and -seronegative subjects (5.3%). HIVseropositive cases who received the extended therapy had a recurrence rate of 1.9% compared with 9% in the placebo group (p < .01). Despite a lower recurrence rate in the HIV seropositive subjects who received extended therapy, there was no difference in survival compared with those who did not receive the extended treatment. The reasons for the higher recurrence rate in the HIV seropositive patients is unknown, but may have been because of nonadherence in the continuation phase or exogenous reinfection. The Tuberculosis Trials Consortium, sponsored by the CDC, conducted a randomized trial of rifapentine versus rifampin in the continuation phase of therapy.(57) Adults who had completed a 2-month induction phase of INH, rifampin, pyrazinamide, and ethambutol were randomly assigned to receive 900 mg INH and 600 mg rifampin twice weekly, or 900 mg INH and 600 mg rifapentine once weekly. Seventy-one HIV-infected patients were enrolled, of whom 61 completed therapy and were assessed for relapse. Five of 30 (17%) patients in the once-weekly INH/rifapentine arm relapsed, compared with three of 31 (10%) patients in the twice-weekly INH/rifampin arm. Four of the five relapses in the once-weekly group had monoresistance to rifampin, compared with none in the standard treatment arm. Enrollment of HIV-infected patients with tuberculosis was halted in the study because of these findings. The authors concluded that HIV-infected patients with tuberculosis should not be treated with a once-weekly INH and rifapentine regimen. In another Tuberculosis Trials Consortium study, an intermittent rifabutin-based regimen was evaluated. This was a single-arm trial of twice-weekly rifabutin-based therapy in HIVinfected persons. Five patients who failed treatment or relapsed developed acquired rifamycin resistance. All five had low CD4 counts (<60 cells/mm3). Four received twiceweekly therapy in the first 2 months of treatment, and all five received twice-weekly therapy in the continuation phase. Based on these findings, for HIV-infected persons with CD4 counts <100 cells/mm3, daily therapy is indicated during the first 2 months, followed by either daily therapy or three doses per week during the continuation phase.(58) Rifampin- vs. Rifabutin-Based Regimens Rifabutin is another rifamycin that is highly active against M tuberculosis. Compared with rifampin, rifabutin shows less induction of hepatic microsomal metabolism. Limited data suggests that rifabutin- and rifampin-based regimens are equally efficacious. A randomized clinical trial in Argentina, Brazil, and Thailand compared rifabutin (at two dosages) with rifampin.(59) A total of 520 HIV-negative patients were enrolled and randomly assigned to receive either rifampin (n = 175), rifabutin 150 mg (n = 174), or rifabutin 300 mg (n = 171). The mean time to culture conversion was similar between groups. There was one relapse in the rifampin group and two in each of the rifabutin groups. A randomized trial in South Africa compared rifabutin to rifampin in a standard four-drug regimen administered in directly observed therapy (DOT).(60) In the continuation phase, the medications were given twice weekly. The mean time to sputum conversion was 14.1 weeks with rifampin versus 14.3 weeks with rifabutin. The difference in relapse rates between the two regimens (3.8% in the rifampin group and 5.1% in the rifabutin group) was not statistically significant. In the only such trial done in HIV-infected patients--a single-blind, randomized study of 50 HIV-infected patients in Uganda--compared a fully supervised regimen of rifampin versus rifabutin together with INH, ethambutol, and pyrazinamide.(61) Time to sputum conversion was similar between groups when controlling for baseline characteristics. Safety and Tolerability The frequency of adverse drug reactions has varied considerably between studies. In two different studies from San Francisco, 18-26% of HIV-seropositive patients with tuberculosis underwent a change in therapy because of adverse drug reactions.(62,63) Rifampin was the drug most commonly implicated, producing an adverse reaction in 12% of the patients. In Zaire, 11% of the seropositive cases developed a rash, but treatment was not interrupted.(52) Paresthesia was common and developed in 21% of cases, pointing out the need for coadministration of pyridoxine (vitamin B6) when INH is used in HIV-1-seropositive individuals. Other investigators have reported low rates of drug reactions.(53,54) Differences between studies in the reported frequency of adverse drug reactions may reflect different patient populations, different degrees of immunosuppression among the patients, and different thresholds of providers to change therapy. Antituberculosis drug-induced hepatotoxicity is common among HIV-infected patients with and without hepatitis C infection. One study found the relative risk of developing druginduced hepatotoxicity in patients with hepatitis C or HIV infection to be fivefold and fourfold, respectively.(64) The relative risk of developing drug-induced hepatitis in patients with both hepatitis C and HIV infection was increased 14-fold. Frequent monitoring of liver function tests during tuberculosis treatment may be indicated in patients with hepatitis C. Thiacetazone, which is used in many developing countries for the treatment of tuberculosis, has been reported to cause frequent and significant skin reactions in HIV-seropositive patients being treated for tuberculosis.(65,66) In one study, up to 20% of the HIVseropositive patients on thiacetazone developed cutaneous rashes, compared with 1% of the seronegative cases; the case-fatality rate was 14% in the HIV-seropositive persons suffering these reactions.(66) The risk of cutaneous reactions in a randomized trial of thiacetazone- and rifampin-containing regimens was nearly 10 times higher in the seropositive cases compared to the seronegative cases.(65) These reports prompted the WHO to abandon thiacetazone in the treatment of HIV-related tuberculosis.(2) Current American Thoracic Society/CDC Treatment Recommendations Recommendations for the treatment of tuberculosis in HIV-infected patients are identical to those for HIV-uninfected cases, with two exceptions: a) once-weekly INH-rifapentine in the continuation phase should not be used; and b) twice-weekly INH-rifampin or rifabutin should not be used for patients with CD4 lymphocyte counts <100 cells/mm3 (Table 3).(67) A 6month (26-week) regimen consisting of INH, rifampin, and pyrazinamide given for 2 months, followed by INH and rifampin for 4 months is the preferred treatment for drugsusceptible organisms (Table 4). Ethambutol or streptomycin should be added to the above regimen until drug susceptibility results are available. In HIV-infected cases, the 6-month regimen should be considered the minimum duration of treatment. It is very important to assess the clinical and bacteriologic response in the setting of HIV-1 infection. Patients with cavitation on initial chest radiograph and positive culture at completion of 2 months of therapy should receive a 7-month (31-week) continuation phase. Antituberculosis therapy that contains INH should be supplemented with pyridoxine (vitamin B6) at 25-50 mg per day to prevent the development of peripheral neuropathy. The most important factor in the treatment of HIV-related tuberculosis is adherence to the treatment regimen. DOT should be considered in all HIV-infected patients. At least one study reported a decreased mortality in HIV-infected patients who received DOT versus selfadministered therapy.(68) Drug-Drug Interactions Successful therapy for tuberculosis requires that HIV-infected individuals take antituberculosis drugs for a minimum of 6 months, in addition to potentially large numbers of other medications. Certain antituberculosis drugs may interact adversely with medications commonly used by HIV-infected individuals. Understanding these drug-drug interactions can prevent drug toxicity and possible treatment failures. The rifamycin derivatives (ie, rifampin, rifabutin, and rifapentine) can induce the hepatic cytochrome P450 enzyme system, resulting in increased metabolism and serum levels of certain drugs.(69) Medications that are known to be affected include fluconazole, ketoconazole, methadone, oral contraceptives, phenytoin, protease inhibitors (PIs), and nonnucleoside reverse transcriptase inhibitors (NNRTIs) (69,70). Coadministration of rifampin with ketoconazole or fluconazole decreases the area under the curve (AUC) of the antifungal by approximately 80% and 20%, respectively.(69) Therefore, ketoconazole and rifampin should not be given together. Rifampin and fluconazole can be used together, but the fluconazole dose may need to be increased. Combination ART is commonly used for the treatment of HIV infection. These agents are divided into nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, and PIs. The rifamycin derivatives accelerate the metabolism of the PIs and NNRTIs resulting in subtherapeutic levels and the potential development of viral resistance to these important agents(70). Of the available rifamycins, rifampin is the most potent inducer of the P450 enzymes and thus produces significant reductions in the serum concentrations of the PIs and NNRTIs. Rifabutin, which is a less potent inducer of the P450 enzymes, can be substituted for rifampin in the treatment regimen. However, because the PIs and NNRTIs affect the metabolism of rifabutin, resulting in altered serum levels and the possibility of drug toxicity, adjustments in rifabutin dosage are often necessary. The CDC recently published pharmacokinetic data regarding the rifamycins and the PIs and NNRTIs (Table 5 and Table 6).(71) These data can be used to design treatment regimens for HIV and tuberculosis. Patients can take the standard rifampin-based treatment regimen if they will not be taking PIs or NNRTIs. Previous guidelines specifically stated that rifampin was contraindicated for patients who were taking any PI or NNRTI.(72) However, new data indicate that rifampin can be used for the treatment of tuberculosis with certain combinations of antiretroviral agents (Table 5 and Table 6).(71) (As discussed above, for patients who are taking PIs or NNRTIs, rifabutin may be substituted for rifampin with proper dose adjustments to rifabutin and the antiretrovirals.) Paradoxical Reaction Some patients may experience a temporary exacerbation of symptoms, signs, or radiographic manifestations of tuberculosis after beginning antituberculosis treatment. This worsening has been referred to as a "paradoxical reaction" and has been noted to occur in HIV-infected patients with active tuberculosis. These reactions often develop after immune reconstitution has occurred in the setting of simultaneous administration of both antiretroviral and antituberculosis medications. A prospective study in Florida found that 36% of HIV patients who were taking antiretroviral agents, compared with 7% who were not, developed a paradoxical worsening of their clinical and/or radiographic condition.(73) The diagnosis of a paradoxical reaction should be made only after a thorough evaluation to exclude other etiologies, such as tuberculosis treatment failure. Symptomatic therapy is sufficient for patients with a paradoxical reaction in whom the symptoms are not severe or life-threatening. For patients whose reactions are associated with severe or life-threatening conditions (eg, uncontrollable fever, airway compromise from enlarging lymph nodes, enlarging serosal fluid collections, sepsis syndrome), management may include the use of corticosteroids. Some experts recommend that prednisone (or equivalent corticosteroid) be started at a dose of 1 mg/kg and reduced after 1-2 weeks based on the resolution of symptoms. Initiation of Antiretroviral Therapy in the Coinfected Patient It is not unusual for the diagnosis of tuberculosis and HIV to be made simultaneously. In this setting, treatment of tuberculosis should be initiated immediately. Whether or when to initiate ART is a more difficult decision. Some experts argue that delaying treatment of HIV infection for 2 months, observing for any adverse effects from the tuberculosis medicines, and potentially decreasing the risk of a paradoxical reaction is a reasonable approach. On the other hand, effective ART can have a significant impact on HIV-related morbidity and mortality. A recent study from Narita and colleagues described the successful use of rifabutin in combination with an antiretroviral regimen containing PIs.(74) All 25 patients became culture negative for M tuberculosis by 2 months of treatment, and no relapses were reported during a median follow-up of 13 months. Moreover, the HIV viral load decreased significantly with 20 of 25 patients achieving viral loads of <500 copies/mL. Thus, it appears that tuberculosis and HIV can be treated concurrently using a rifabutin-based regimen. In patients already receiving ART, the regimen should be continued, and modifications to either the tuberculosis regimen or to the antiretroviral regimen can be made as indicated. Whether it is best to start ART as soon as possible or wait until antituberculosis treatment is well established is not clear at present, so the decision should be made on a case-by-case basis, taking into account factors related to both adherence (such as motivation and stability of living situation) and potential for complications (such as clinically active hepatitis C). Involvement of the patient in the decision of whether and when to start ART is crucial. It is important to be aware of the potential problems that can occur when antituberculosis medications and antiretroviral agents are administered concurrently. Gastrointestinal complaints and rash are not uncommon with antituberculosis medications and these can be quite common with certain antiretroviral medications. A flulike illness has been described with rifampin, which could be confused with an abacavir hypersensitivity reaction. Peripheral neuropathy is an adverse effect of INH as well as stavudine and didanosine. Elevated liver function tests can occur with INH, rifampin, pyrazinamide, and most of the NRTIs, NNRTIs, and PIs. It can be challenging to determine which medication is the offending agent. In the setting of tuberculosis, this usually involves holding all medications and restarting sequentially to determine which medication caused the problem. Although this approach works quite well with tuberculosis medications, in the case of HIV infection, sequential addition of antiretroviral drugs is not advisable because of the risk of developing antiretroviral resistance. When an adverse reaction occurs with antiretroviral medications, the agent most likely to be responsible is usually deduced based on known adverse-effect profiles and clinical judgement. Diagnosis and Treatment of Latent Tuberculosis Infection Diagnosis of Latent Tuberculosis Infection Screening for latent tuberculosis infection (LTBI) is an essential step in controlling the spread of tuberculosis. Screening for LTBI is recommended in persons at risk for recent infection and in those groups with increased risk of progression to active disease once infected, including HIV-infected persons. The tuberculin skin test (Mantoux method) is currently the only method available for identifying LTBI. Routine annual tuberculin skin testing is recommended in HIV-infected individuals. A reaction of >=5 mm induration is considered positive for HIV-infected patients and persons with other forms of severe immunosuppression, persons who are close contacts of infectious cases, and persons with abnormal chest radiographs consistent with tuberculosis.(75). Use of the 5-mm cutoff is supported by a prospective study in the United States demonstrating that the risk of tuberculosis was significantly higher in HIV-infected persons with tuberculin skin test reactions >=5 mm of induration than in those who have a reaction <5 mm.(25) It is important to keep in mind that a negative tuberculin skin test does not exclude infection or active disease. Testing with tuberculin purified protein derivative is dependent on the presence of an intact cell-mediated immune response. In the setting of HIV infection, reduced cell-mediated immunity can lead to decreased delayed-type hypersensitivity (DTH) responsiveness, resulting in false-negative skin tests. In a multicenter study in the United States, the prevalence of a positive tuberculin skin test (>=5 mm) was shown to decrease with decreasing CD4 T-cell counts.(76) Persons who are at risk for tuberculous infection (eg, injection drug users, individuals who are institutionalized or from high-prevalence regions) should have a chest radiograph performed even if the tuberculin skin test is negative, particularly if their CD4 T-cell count is low. Annual chest radiographs should be considered in this high-risk group. Application of multiple skin test antigens (eg, Candida, mumps, tetanus toxoid, etc.), referred to as anergy testing, has been used to assess cell-mediated immune function and to distinguish true-negative from false-negative tuberculin skin test results. In 1991, the CDC recommended that anergy testing be performed in conjunction with tuberculin skin testing in HIV-infected persons based on the premise that anergic HIV-infected individuals at high risk for tuberculosis infection would benefit from treatment with INH.(77) In 1997, the CDC revised its recommendations and no longer recommends anergy testing while screening for M tuberculosis infection in HIV-infected persons.(78) The revised recommendation is based on the following points. First, there are no standardized guidelines for performing anergy skin testing. The appropriate number of control antigens to administer or the appropriate cut-off for interpreting a test as positive is not known. Second, the response to skin testing with control antigens as well as with tuberculin can vary over time. Several studies have demonstrated that HIV-1-seropositive individuals can regain DTH responsiveness with time.(29,72,79) In a multicenter study, Chin and colleagues(79) reported that 31% of anergic HIV-1-seropositive patients responded to DTH testing 1 year later. The only factor associated with regaining DTH responsiveness was the CD4 T-cell count: the higher the CD4 count, the more likely the individual was to regain DTH responsiveness. Finally, treatment of LTBI in anergic HIV-infected persons has not been demonstrated to be beneficial.(80,81) In some individuals with tuberculous infection, DTH responsiveness may decrease with time. A second tuberculin skin test, applied weeks to months after the first, can "boost" the DTH response resulting in a positive skin test reaction. Such responses are considered true evidence of tuberculous infection. In a multicenter study in the United States, only 2.7% of HIV-1-seropositive patients "boosted" the tuberculin reaction with a second tuberculin skin test, despite relatively high demographic risk of tuberculous infection.(82) However, a study in Uganda found that 17 (29%) of 58 HIV-1-infected subjects responded to a second tuberculin skin test.(83) Because most groups in the United States have a relatively low prevalence of tuberculosis, two-step tuberculin skin testing is not recommended routinely. Treatment of Latent Tuberculosis Infection HIV-infected individuals with LTBI have an extraordinarily high rate of progression to active tuberculosis compared to HIV-uninfected persons. Fortunately, treatment of LTBI is very effective in preventing persons infected with M tuberculosis from developing active disease. Annual screening of all HIV-infected persons for LTBI, and treatment of those coinfected with M tuberculosis, is therefore recommended. Treatment regimens for LTBI are not adequate for the treatment of active tuberculosis, and may select for drug-resistant strains if inadvertently used in the setting of active disease. Therefore, after a positive skin test, active tuberculosis must be ruled out before providing treatment for latent infection. There have been two placebo-controlled trials with INH in tuberculin-positive, HIV-infected patients (Table 7).(39,81) Investigators in Haiti reported the results of the first randomized, placebo-controlled study of INH given for 6 months.(39) Treatment with INH significantly reduced the rate of tuberculosis compared with placebo (10.0 vs. 1.7 per 100 person-years) in tuberculin-positive individuals. A placebo-controlled study in Uganda compared four treatment arms: placebo for 6 months, INH for 6 months, INH and rifampin for 3 months, and INH, rifampin, and pyrazinamide for 3 months.(81) As shown in Table 7, all of the treatment arms significantly reduced the rate of tuberculosis compared with placebo. Three prospective, randomized trials have compared 2-3 months of the dual-drug regimen rifampin plus pyrazinamide to 6-12 months of INH.(84-86) The most recent of these trials demonstrated that 2 months of daily rifampin and pyrazinamide was as effective as 12 months of daily INH in HIV-infected persons.(86) Studies have also found intermittent dosing of INH,(84,85) or pyrazinamide plus rifampin,(84,85) to be effective, but intermittent dosing has not been directly compared with daily regimens. Current American Thoracic Society/CDC Recommendations Until recently, a 6- or 12-month course of INH was the recommended regimen for treatment of LTBI in HIV-seronegative and -seropositive persons, respectively. The current guidelines from the American Thoracic Society and CDC offer three options for the treatment of LTBI in HIV-infected persons based on the studies described above (Table 8).(75) The first option is to give INH daily or twice weekly for 9 months instead of the previous recommendation of 12 months. This new recommendation is generalized from data in HIV-uninfected persons that 12 months of INH is more efficacious than 6 months of therapy in preventing active tuberculosis but that minimal benefit is gained by extending treatment from 9 to 12 months.(87) The second option for treatment of LTBI is rifampin plus pyrazinamide administered daily for 2 months. However, since the above guidelines were published, 21 cases of liver injury, including five deaths, have been reported in HIV-uninfected persons receiving a 2-month regimen of rifampin and pyrazinamide. The American Thoracic Society and CDC have prepared new recommendations given these unexpected findings. For HIVuninfected persons, 9 months of INH is the preferred regimen. Four months of daily rifampin would be the next alternative (discussed below). The 2-month rifampin-pyrazinamide regimen should be used with caution, only in patients who can be closely monitored and for whom the likelihood of completing a longer treatment course is unlikely. Even though trials of 2-3 months of rifampin and pyrazinamide in HIV-infected persons did not show increased risk of severe hepatitis, it may be advisable to use 9 months of INH until more is known about the frequency of, and risk factors for, toxicity in HIV-infected persons.(88) The third regimen for treatment of LTBI is rifampin daily for 4 months. Rifampin alone for 3 months was shown to be more efficacious than placebo in HIV-negative persons with silicosis.(89) However, because the rate of active TB was still high at 10%, the recommendation is to extend the duration of therapy to 4 months for both HIV-infected and -uninfected persons who cannot tolerate INH or pyrazinamide. Although there are no studies of rifabutin-containing regimens in the treatment of LTBI, rifabutin appears to be equal in efficacy to rifampin in the treatment of tuberculosis. Therefore, rifabutin can be substituted for rifampin in cases where rifampin is contraindicated. An INH-containing regimen should be supplemented with pyridoxine (25-50 mg/day) to prevent the development of peripheral neuropathy. HIV-infected individuals who are close contacts of persons with infectious tuberculosis should receive treatment for LTBI regardless of the results of the tuberculin skin test, as long as active tuberculosis has been ruled out.(90,75) In addition, because exogenous reinfection has been demonstrated in patients with AIDS, treatment should be given even if a prior course of therapy for tuberculosis or LTBI has been completed.(33) Contacts of known cases of INH-resistant tuberculosis should be treated with rifampin (600 mg/day) instead of INH. Close contacts to MDRTB cases should be treated with at least two drugs to which the source isolate is susceptible. References 1. AIDS Control and Prevention (AIDSCAP) Project of Family Health Internal, The Francois-Xavier Bagnoud Center for Public Health and Human Rights Of the Harvard School of Public Health, UNAIDS. The Status and Trends of the Global HIV/AIDS Pandemic. Final Report July 5-6, 1996. 2. Raviglione MC, Narain JP, Kochi A. HIV-associated tuberculosis in developing countries: clinical features, diagnosis, and treatment. Bull WHO 1992;70:515-526. 3. Raviglione MD, Snider DE, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA 1995;273:220-226. 4. Small PM. Tuberculosis research. Balancing the portfolio. JAMA 1996;276:1512-1513. 5. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA 1999;282:677-686. 6. World Health Organization. Map available at http://www.who.int/gtb/publications/globrep00/PDF/GTBR2000annex4.pdf 7. Centers for Disease Control and Prevention. Tuberculosis morbidity - United States, 1992. Morb Mortal Wkly Rep 1993;42:696-704. 8. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 1999. August, 2000, pp 1-95. 9. Brodt HR, Kamps BS, Gute P, et al. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS 1999;11:1731-1738. 10. Ole K, Gatell JM, Mocroft A, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active retroviral therapy. Am J Respir and Crit Care Med 2000:162:867-872. 11. Bloch AB, Cauthen GM, Onorato IM, et al. Nationwide survey of drug-resistant tuberculosis in the United States. JAMA 1994;271:665-671. 12. Centers for Disease Control and Prevention. Nosocomial transmission of multidrug resistant tuberculosis among HIV-infected persons - Florida and New York, 1988-1991. Morb Mortal Wkly Rep. 1991;40:585591. 13. Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen G, Dooley SW. The emergence of drug resistant tuberculosis in New York City. N Engl J Med 1993;328:521-526. 14. Gordin FM, Nelson ET, Matts JP, et al. The impact of human immunodeficiency virus infection on drugresistant tuberculosis. Am J Respir Crit Care Med 1996;154:1478-1483. 15. Nolan CM, Williams DL, Cave MD, et al. Evolution of rifampin resistance in human immunodeficiency virus-associated tuberculosis. Am J Respir Crit Care Med 1995;152:1067-71. 16. Bishai WR, Graham NM, Harrington S, et al. Brief report: rifampin-resistant tuberculosis in a patient receiving rifabutin prophylaxis. N Engl J Med 1996;334:1573-1576. 17. Bradford WZ, Martin JN, Reingold AL, Schecter GF, Hopewell PC, Small PM. The changing epidemiology of acquired drug-resistant tuberculosis in San Francisco, USA. Lancet 1996;348:928-931. 18. Luftey M, Della-Latta P, Kapur V, et al. Independent origin of mono-rifampin-resistant Mycobacterium tuberculosis in patients with AIDS. Am J Respir Crit Care Med 1996;153:837-840. 19. Alland D, Kalkut GE, Moss AR, et al. Transmission of tuberculosis in New York City: An analysis by DNA fingerprinting and conventional epidemiologic method. N Engl J Med 1994;330:1710-1716. 20. Small PM, Hopewell PC, Singh SP, et al. The epidemiology of tuberculosis in San Francisco: a populationbased study using conventional and molecular methods. N Engl J Med 1994;330:1703-1709. 21. Edwards D, Kirkpatrick GH. The immunology of mycobacterial diseases. Am Rev Respir Dis 1986;134:1062-1071. 22. Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Ann Rev Immunol 1990;8:169194. 23. Hopewell PC, Bloom BR. Tuberculosis and other mycobacterial diseases. In: Murray JF, Nadel JA, eds. Respiratory Medicine, 3rd ed. Philadelphia, PA, WB Saunders Company, 2000:1043-1105. 24. Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction fragment length polymorphism. N Engl J Med 1992;326:321-335. 25. Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIVinfected persons. Ann Intern Med 1997;126:123-132. 26. Selwyn P, Hartel D, Lewis V, et al. A prospective study of the risk of tuberculosis among intravenous drug users with HIV infection. N Engl J Med 1989;320:545-550. 27. Selwyn P, Sckell B, Alcabes P, Friedland G, Klein R, Shoenbaum E. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA 1992;268:504-509. 28. Moreno S, Baraia-Etxaburu J, Bouza E, et al. Risk for developing tuberculosis among anergic patients infected with HIV. Ann Intern Med 1993;119:194-198. 29. Antonucci G, Girardi E, Raviglione MC, Ippolito G, for the Gruppo Italiano di Studio Tuberculosi e AIDS (GISTA). Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. JAMA 1995;274:143-148. 30. Daley CL, Hahn JA, Moss A, Hopewell PC, Schecter GF. Incidence of tuberculosis in injection drug users in San Francisco. Am J Respir Crit Care Med 1998:157;19-22. 31. Nardell E, McInnis B, Thomas B, Weidhaas S. Exogenous reinfection with tuberculosis in a shelter for the homeless. N Engl J Med 1986;315:1570-1575. 32. Shafer RW, Singh SP, Larkin C, Small PM. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in an immunocompetent patient. Tuberc and Lung Dis 1995;76:575-577. 33. Small PM, Shafer RW, Hopewell PC, et al. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med 1993;328:1137-1144. 34. Horn DL, Hewlett D, Haas WH, et al. Superinfection with rifampin-isoniazid-streptomycin-ethambutol (RISE)-resistant tuberculosis in three patients with AIDS: Confirmation by polymerase chain reaction fingerprinting. Ann Intern Med 1994;121:115-116. 35. Godfrey-Faussett P, Stoker NG. Aspects of tuberculosis in Africa. 3. Genetic fingerprinting for clues to the pathogenesis of tuberculosis. Trans Royal Soc Trop Med and Hygiene. 1992;86:472-475. 36. Hawkens M, Nunn P, Gathua et al. Increased recurrence of tuberculosis in HIV-1 infected patients in Kenya. Lancet 1993;342:332-337. 37. Osset J, Martin-Casabona N, Bellver P, Xairo D, Codina G. Recurrences of tuberculosis in HIV related patients. Period 1985-94. Tuberc and Lung Dis 1995;76:137. 38. De Cock KM, Soro B, Coulibaly IM, Lucas SB. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA 1992;268:1581-1587. 39. Pape JW, Jean SS, Ho JL, Hafner A, Johnson DW. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet 1993;342:268-272. 40. Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. J Immunol 1996;157:1271-1278. 41. Wallis RS, Vjecha M, Amir Thamasseb M, et al. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated beta 2-microglobulin HIV-1 associated tuberculosis. J Infect Dis 1993;167:43-48. 42. Shattock RJ, Friedland JS, Griffin GE. Modulation of HIV transcription in and release from monocytic cells following phagocytosis of Mycobacterium tuberculosis. Res Virol 1993;144:7-12. 43. Lederman MM, Georges D, Kusner DJ, Mudido P, Giam CZ, Toossi Z. Mycobacterium tuberculosis and its purified protein derivative activate expression of the human immunodeficiency virus. J AIDS 1994;7:727733. 44. Zhang Y, Nakata K, Weiden M, Rom WN. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest 1995;95:2324-2331. 45. Jones BE, Young SMM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis 1993;148:1292-127. 46. Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+ T-lymphocyte count. Tubercle and Lung Dis 1995;76:518-21. 47. Greenberg SD, Frager D, Suster B, et al. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance). Radiology 1994;193:115-119. 48. Long R, Maycher B, Scalcini, M et al. The chest roentgenogram in pulmonary tuberculosis patients seropositive for immunodeficiency virus type-1. Chest 1991;99:123-127. 49. Gary SM. Tuberculosis and the Human Immunodeficiency Virus. In: Rom and Garay, eds. Tuberculosis, 1st ed. New York, Little, Brown, and Company, 1996. p 451. 50. Kennedy DJ, Lewis WP, Barnes PF. Yield of bronchoscopy for the diagnosis of tuberculosis inpatients with human immunodeficiency virus infection. Chest 1992;102:1040-1044. 51. Centers for Disease Control and Prevention. Update: Nucleic Acid Amplification Tests for Tuberculosis. Morb Mortal Wkly Rep 2000;49:593-594. 52. Perriens JH, St. Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. New Engl J Med 1995; 332:779-784. 53. Chaisson RE, Clermont HC, Holt EA, et al. Six-month supervised intermittent tuberculosis therapy in Haitian patients with and without HIV infection. Am J Respir Crit Care Med 1996;154:1034-1038. 54. El-Sadr, WM, Perlman, DC, Matts, JP, et al. Evaluation of an intensive intermittent-induction regimen and duration of short-course treatment for human immunodeficiency virus-related pulmonary tuberculosis. Clin Infect Dis 1998; 26: 1148-1158. 55. Kassim, S, Sassan-Morokro, M, Ackah, A et al. Two year follow-up of persons with HIV-1 and HIV-2 associated pulmonary tuberculosis treated with short course chemotherapy in West Africa. AIDS 1995; 9: 1185-1191. 56. Kennedy, N, Berger, L, Curram, J et al. Randomized controlled trial of a drug regimen that includes ciprofloxacin for the treatment of pulmonary tuberculosis. Clin Infect Dis 1996; 22: 827-833. 57. Vernon, A., Burman, W, Benator, D, Khan, A, Bozeman, L for the Tuberculosis Trials Consortium. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet 1999:353;1843-1847. 58. Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin-based regimens. MMWR Morb Mortal Wkly Rep. 2002 Mar 15;51(10):214-5. 59. Gonzalez-Montaner, Natal S, Yonchaiyud P, et al. Rifabutin for the treatment of newly-diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus rifampicin. Tuber Lung Dis 1994;75:341-347. 60. McGregor MM, Olliaro P, Womarans L, et al. Efficacy and safety of rifabutin in the treatment of patients with newly diagnosed pulmonary tuberculosis. Am J Respir Crit Care Med 1996;154:1462-1467. 61. Schwander S, Rusch-Gerdes S, Mateega A, et al. A pilot study of antituberculosis combinations comparing rifabutin in the treatment of patients with newly diagnosed pulmonary tuberculosis. Tuber Lung Dis 1995;76:210-218. 62. Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis 1987;136:570-574. 63. Small PM, Schecter GF, Goodman PC, Sande MA, Chaisson RE, Hopewell PC. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med 1991;324:289-294. 64. Ungo JR, Jones D, Ashkin D, et al. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med 1998;157:1871-1876. 65. Okwera A, Whalen C, Byekwaso F, et al. Randomized trial of thiacetazone and rifampicin-containing regimens for pulmonary tuberculosis in HIV infected Ugandans. Lancet 1994;344:1323-1328. 66. Nunn P, Kibuga D, Gathua S, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet 1991;337:627-630. 67. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003 Feb 15;167(4):603-62. 68. Alwood K, Keruly JC, Morrore-Rice K, Stanton DL, Chaulk P, Chaisson RE. Effectiveness of supervised, intermittent therapy for tuberculosis in HIV-infected patients. AIDS 1994;8:1103-1108. 69. Borcherding SM, Baciewicz AM, Self TH. Update on rifampin drug interactions II. Arch Intern Med 1992;152:711-716. 70. Centers for Disease Control and Prevention. Clinical Update: Impact of HIV protease inhibitors on the treatment of HIV-infected tuberculosis patients with rifampin. Morb Mortal Wkly Rep 1996;45:921-925. 71. Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep 2000;49:185-200. 72. Caiaffa WE, Graham NMH, Galai N, Rizzo RT, Nelson KE, Vlahov D. Instability of delayed-type hypersensitivity skin test anergy in human immunodeficiency virus infection. Arch Intern Med 1995;21112117. 73. Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998;158:157-161. 74. Narita M, Stambaugh JJ, Hollender ES, Jones D, Pitchenik AE, Ashkin D. Use of rifabutin with protease inhibitors for human immunodeficiency virus-infected patients with tuberculosis. Clin Infect Dis 2000;30:779-783. 75. Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Morb Mortal Wkly Rep 2000;49:1-51. 76. Markowitz N, Hansen N, Wilcosky T, et al. Tuberculin and anergy skin testing in HIV seropositive and HIV seronegative persons. Ann Intern Med 1993;119:185-93. 77. Centers for Disease Control. Purified protein derivative (PPD)-tuberculin anergy and HIV infection. Morb Mortal Wkly Rep 1991;40:27-33. 78. Centers for Disease Control and Prevention. Anergy Skin Testing and Preventive Therapy for HIV-Infected Persons: Revised Recommendations. Morb Mortal Wkly Rep 1997;46(No.RR-15):1-10. 79. Chin DP, Osmond D, Page-Shafer K, et al. Reliability of anergy skin testing in persons with HIV infection. Am J Respir Crit Care Med 1996;153:1982-1984. 80. Gordin FM, Matts JP, Miller C, et al. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med 1997;337:315-320. 81. Whalen CC, Johnson JL, Okwera A, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med 1997;337:801-808. 82. Webster CT, Gordin FM, Matts JP, et al. Two-stage tuberculin skin testing in individuals with human immunodeficiency virus infection. Am J Respir Crit Care Med 1995;151:805-808. 83. Hecker MT, Johnson JL, Whalen CC, Nyole S, Mugerwa RD, Ellner JJ. Two-step tuberculin skin testing in HIV-infected persons in Uganda. Am J Respir Crit Care Med 1997;155:81-86. 84. Halsey NA, Coberly JS, Desormeaux J, et al. Randomized trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet 1998;352:786-792. 85. Mwinga AM, Godfrey-Faussett P, Quigley M, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS 1998;12:2447-2457. 86. Gordin FM, Chaisson RM, Matts JP, et al. An international, randomized trial of rifampin and pyrazinamide versus isoniazid for prevention of tuberculosis in HIV infected persons. JAMA 2000;283:1445-1450. 87. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;10:847-850. 88. Update: Fatal and severe liver injuries associated with rifampin and pyrazinamide for latent tuberculosis infection, and revisions in American Thoracic Society/CDC recommendations--United States, 2001. MMWR Morb Mortal Wkly Rep. 2001 Aug 31;50(34):733-5. 89. Hong Kong Chest Service, Tuberculosis Research Centre, Madras, and British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41. 90. Centers for Disease Control and Prevention. Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. Morb Mortal Wkly Rep 1998;47:1-58.