Landscape Paper 2003 final - V2_MBedits

Patch Dynamics and Seed Dispersal:
Implications for Restoration and
Conservation
Shannon Galbraith
INTRODUCTION
Habitat alteration and destruction by humans has occurred for centuries. The settlement of Europeans in North America, along with the extensive use of fire by Native
Americans, began a trend of deforestation and forest fragmentation on this continent. What has resulted is a distribution of patches in the landscape. Many patches are remnants of the original habitat. A patch suggests an area that is relatively discrete in space (White and Pickett 1985).
Not constrained by size, area, persistence, composition, or geographic location, patches are areas that are different than the landscape matrix, depending on the process or pattern of interest (Turner et al. 2001). Just as scale differs with the system being studied (Wiens 1989), it cannot be over-emphasized that characteristics of patches are neither generalizable, nor static.
Patches are dynamic through time and space. Patch dynamics is a process across a landscape, in which a changing patchwork is created from disturbances differing in frequency
( e.g., seasonally), size, type ( e.g., abiotic, biotic, human), shape, and intensity (Parker and
Pickett 1998 in Turner et al. 2001). The effects of patch dynamics are evident in changing landscapes, as various patterns ( e.g., natural plant communities) at very different scales ( e.g., annual herbs, biome) are altered. And, as patches change, associated processes such as seed dispersal are predicted to also change. The configuration of landscape patches will directly impact the ability of plant populations to colonize and persist, as seeds are often dispersed into patches ( e.g., forest gaps, savanna tree islands, ant colonies) by bird defecation, wind, ants, and other dispersal modes.
Seed dispersal is a crucial process in the maintenance and establishment of plant populations (Griffith and Forseth 2002), and is how individual patches of plants remain interconnected, functioning as a unique dynamic system (Valverde and Silvertown 1997). Many plant populations are distributed patchily at some scale, illustrating the heterogeneity of landscape habitat and the patterns of disturbance and seed recruitment (White and Pickett
1985). Dynamic characteristics such as patch configuration and size will influence short-term factors, including the demography of plant populations ( e.g., density, distribution). For example, depending on where plants are distributed, the mode of dispersal ( e.g., bird), and plant abundance will impact how dispersal evolves for that species over a longer period of time
(Gadgil 1971, Alvarez-Buylla et al. 1996).
A decrease in recruitment is thought to be one of the primary reasons that cause extinction of local plant populations in fragmented, or patchy, landscapes (Bruna 2003). In these landscapes, seeds must disperse through a lattice of patches that are “safe” and “unsafe”;
2 seeds are more likely to be recruited at “safe sites” where the abiotic ( e.g., light) and biotic
( e.g., lack of predators) requirements for successful establishment exist (Harper 1977, Orth et al.
2003). As the landscape changes, the proportion and spatial arrangement of “safe sites” may change for a given plant population, thus affecting the number of seeds that are able to germinate and be recruited. For example, if a landscape is fragmented in a way that increases patches occupied by seed predators ( i.e., increase in unsafe sites), then the probability of seedling recruitment may decrease as a result.
In this review of literature, the primary methods used to identify seed dispersal in relation
to patch dynamics will be discussed, as well as the importance of patch configuration in a
landscape for dispersal and in particular, long-distance dispersal , and how patch dynamics and
seed dispersal are important co-factors in restoration and conservation ecology .
MEASURING SEED DISPERSAL
Overall, our ability to understand the effects of patch dynamics on seed dispersal depends on accurately counting the movement of seeds across a given landscape and recognizing how habitat patch characteristics ( e.g., size, configuration in the landscape) affect dispersal. To do so, it is essential there is demographic knowledge of the species in question, such as the probability that an individual in class-size j will flower, the total number of fruits produced by individuals of class-size j , and the number of seeds per fruit (Wang and Smith
2002, Bruna 2003). Unfortunately, since so many species have yet to be described, this information is not always obtainable. However, advances are being made, and techniques such as paternity analyses help determine the effect of proximity for seedlings in patches (Bruna
2003).
To study patch dynamics and seed dispersal, three primary methods exist: field observation, experimentation, and modeling . Many studies use all three. For example, the immense scale of most landscapes makes direct field measurement challenging, and therefore, studies may incorporate field observations and census data ( e.g., Bruna 2003) into a computer model
(Turner et al. 2001).
While some studies have occurred in actual landscapes (Hewitt and Kellman 2002 b , Orth et al. 2003), most have used simulation models that incorporate empirical data from (1) plant populations in the patches and the (2) matrix of the species of interest. The matrix includes “background” habitat that is not optimal for a given organism. By holding plant demographic parameters ( e.g., density, distribution) constant, while varying landscape variables ( e.g., patch size, distance to nearest patch), the effects of landscape change on seed dispersal and
3 population persistence can be tested. In 2002, He et al. investigated the effects of humans on the recovery of Korean pines ( Pinus koraiensis ). By using LANDIS model (see He et al. 1999), a forest landscape change simulation model, they found that the direct and indirect actions of humans ( e.g., decreases in habitat patch size) may be producing long-term alterations to forest patch structure. The changes in forest structure would consequently inhibit pine recruitment.
For example, in their model, even if a complete natural succession occurred (~300 yrs) in this system, Korean pine would only persist on one-third of the lands it can potentially dominate.
Because the individuals of this species are usually slow to establish in an area and produce seeds, the lack of seed source patches (“source strength”) exacerbated by land-use change could limit the population’s ability for recovery across the landscape. Studies like these can give us information for policy-making and for management in areas such as, conservation and restoration.
In other modeling studies, landscape variables may be held constant, while the plant characteristics are altered. For example, in a California river system, Levine (2003) used a simulation model to alter the direction of dispersal for perennial plant populations. As a result, he found species coexistence increases when dispersal is primarily one-way. Downstream, species diversity and competition was highest, but because the inferior competitors could find refuge in the upstream neighborhoods, they were not eliminated. In this model, changing how seeds were dispersed to patches (here, tussocks in small rivers) helped explain how these species are able coexist across a landscape.
LONG-DISTANCE DISPERSAL (LDD) AND IMPACTS ON CONSERVATION
AND RESTORATION
As habitat fragmentation and global warming increases with time, the ability for temperate species to extend their ranges and persist will greatly depend on patch connectance and long-distance dispersal (hereafter, LDD) in a landscape. LDD includes events in which propagules arrive, but do not necessarily establish, at patches that are “far removed” from the original source (Nathan et al. 2003). Because “far removed” can vary with the study system
( e.g., riparian herbs, oak trees), LDD continues to be difficult to define (Nathan et al. 2003).
Studies have modeled LDD as distances from 80-meters for nonwoody plants (Bullock and
Clark 2000) to 1600-meters for tree species (Greene and Johnson 1995).
Stepping-stone dispersal and LDD are essentially the same, with the only difference being that the former is a shorter distantce (MOBOT 2003). Both rely on the distribution of habitat patches to facilitate seed movement from the origin ( i.e., seed source) to a distant patch, then to a more-distant patch, and so forth. Patch dynamics play a particularly important role in these types of dispersal, because the landscape scale is typically larger (Nathan et al.
2003), landscape configuration of patches becomes important, and it is plausible more patches would be necessary for dispersal over space and time.
4
Forested ecosystems have been commonly used to study LDD. Generally, it is predicted that a change in the disturbance regime that increases the number of patches (and light and nutrient availability) can increase not only the density of seedlings, but also the local range of a population. Seeds produced in an undisturbed understory remain there, while seeds in patchy forests with gaps tend to be scattered across longer distances (Cipollini et al. 1994).
Studying the dispersal of the shade-tolerant understory shrub, Lindera benzoin (spicebush),
Cipollini et al. (1994) found that the proportion of seeds in gaps to those in the understory increased primarily due to LDD. Thus, by providing “stepping stones” in the understory matrix, the gaps were facilitating LDD. Similarly, Valverde and Silvertown (1997) tested the assumption that the gap-colonizing herb, Primula vulgaris , would have a positive relationship between seed dispersal (and population growth) and increased gap frequency. What they found was the opposite, as there was a negative effect of LDD on the population. Their model predicted that the greater proportion of seeds dispersed out of occupied patches would lower the population growth rate. This latter study is a good illustration of a possible consequence of LDD, where the number of seeds dispersed into unsafe sites ( i.e., non-gap patches with low light) increases, resulting in decreased population growth. Therefore, the effects of LDD on seed dispersal and consequential population growth, or loss, will depend on the species’ tolerance of various biotic and abiotic conditions ( e.g., light in these scenarios). And, the changes in patch characteristics will directly affect these conditions in a landscape.
The affects animals have on seed dispersal are numerous and have been documented in systems where ants ( e.g., Ruhren and Handel 2003), birds ( e.g., Jordano and Schupp 2000), tapirs (Fragoso et al. 2003), and other organisms, are necessary for seed dispersal. As fragmentation increases with urbanization, the roles of animals as means for dispersing seeds across a landscape will become more important (Hewitt and Kellman
2002 a ). For example, in areas of southern Africa where human encroachment is increasing, the presence of naturally sparsely scattered
Acacia erioloba trees in the arid savanna maintain the plant and animal community structure through patch dynamics (Dean et al. 1999). Each individual tree is a patch where frugivorous birds perch and mammals rest, eat, and defecate.
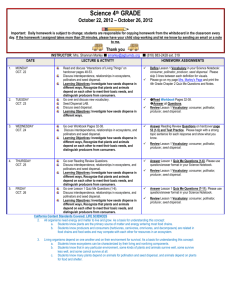
Consequently, seeds are dispersed at long distances across the savanna. The high amount of soil nutrients beneath the trees, from defecation and fallen nest material, increases seedling recruitment. As a result, plants with fleshy-fruits are positively correlated with the distribution of the live and dead Acacia trees in the landscape (Fig. 1). In Figure 1, wind-dispersed plants are found within the matrix with no association with Acacia trees or saplings, but the majority of frugivore-dispersed plants rely on the presence of the trees for their persistence. Thus, the current integrity of an arid savanna depends on patchiness (nutrient-rich shaded tree islands), which increases biodiversity in an otherwise severe and nutrient-poor system. Removing these trees (dead and alive) and creating a homogenous landscape, lacking any patch dynamics, would cause losses of the animals and plants specific to this system. Therefore, there is great need to conserve the patches ( i.e., individual trees) in this landscape, which affect not only the birds and mammals, but also the plant populations.
5
In Brazil , the persistence of the palm, Maximiliana maripa is increased through LDD by tapirs (Fragoso et al. 2003). Predated by bruchid beetles, these palm seeds benefit from tapirs, as they are wrapped in fecal material and carried relatively far where beetles are not present.
Thus, many of the palm seeds that survive as seedlings are found in patches long distances from the original seed source. In this system, LDD by tapirs can result in an almost complete escape of the palm seeds from predation by beetles. As tapirs alter their habitat usage and the patches they frequent, the distribution of patches of palm populations will similarly change.
Figure 1.
Types of seed dispersal to patches of Acacia erioloba trees and matrix vegetation, and consequential densities of herbaceous plants (ha -1), in a grassland landscape, Kalahari Desert, Africa (adapted from Appendix 1 in Dean et al.1999
).
MATRIX
2250
DEAD TREE
1500
750
0 SAPLING
LIVE TREE
Explosive dehiscence
Herbivore-dispersed
Wind-dispersed
Frugivore-dispersed
THE LINKAGE BETWEEN RESTORATION AND CONSERVATION
The idea of “if you create a habitat, colonists will come” will apply to some species more than others (Palmer et al. 1997), and will depend on many factors including, patch connectivity
(Borgella et al. 2001, Tewksbury et al. 2001), the inherent rarity of the species (Maina and Howe
2001, Bruna 2003), and immediate seed sources (Huxel and Hastings 1999, Robinson and
Handel 2000).
Through patch dynamics at the landscape level, there is a strong linkage between restoration ecology and conservation ecology. To think of restoration and conservation today as independent processes is unwise (Dobson et al. 1997). The amount of habitat that is lost prior
6 to abandonment will typically influence the ability of populations and communities to reestablish or be restored. It is reasonable to conclude that if there are limited sources of seeds then the potential of new seedling recruits will be limited as well. Velland (2003) stated that the loss of ancient-forests, which contain strong seed sources, has negatively impacted the recovery of herbaceous plant diversity in post-agricultural forests. In the Chesapeake Bay, Orth et al.
(2003) stressed the importance of conserving eelgrass ( Zostera marina ) seed banks. With these banks conserved seeds are dispersed from habitat patches on rafting shoots, the seed supply may be the primary determinant in how many un-vegetated patches become restored with Zostera . In the context of conserving seed sources, whatever remnant patches can be preserved (regardless of size) will help restore populations that are close in proximity. As habitat patches become more fragmented and proximate seed sources unavailable, the ability of a species to disperse across greater distances ( i.e., LDD) may become important for its persistence.
One of the main questions in conservation is, which habitats will have the greatest benefit to plants and animals: those with large areas or habitat that is connected by a corridor
(Tewksbury et al. 2002, Wood and Pullin 2002)? And rather than asking how much habitat should be restored, the most important corollary decision in restoration is, which habitat should be restored ( e.g., Huxel and Hastings 1999)? As stated above, any patch that can be preserved to be used as potential seed sources is beneficial to plant persistence (Orth et al. 2003, Velland
2003). In restorations, it is important to know the dispersal abilities of the plants while designing restoration plans (Pakeman et al. 2002) and the closer the patches containing target plant species are to the restoration site, the greater chance seeds will be dispersed ( e.g., by birds) and established (Robinson and Handel 2000). By carefully choosing which habitats to restore, based on adjacency or proximity to seed sources of target species, restoration projects can have a high rate of success. And, since many restorations are limited on funds and available sites, the potential success is especially important (Huxel and Hastings 1999).
CONCLUSION
Patches are areas across a landscape that differ from the dominant matrix. The ways in which these patches change in space and time make them dynamic in nature, along with many of the associated processes and patterns. One of these processes is seed dispersal, and the modes and quantity of dispersal will directly affect the pattern of plant populations in a landscape. As fragmentation increases with urbanization, it is important to recognize how patches play key roles ( e.g., pollination, seed sources) in conserving and restoring native plant
7 populations and communities. Doing so will be especially needed at the landscape-level, as our own activities continue to change habitat distributions. By preserving remnant habitats that are connected, successful restorations can be done in proximate locations, as seeds can be dispersed from the remnant patches.
LITERATURE CITED
AlvarezBuylla, E.R., Á. Chaos, D. Piňero, and A.A. Garay. 1996. Demographic genetics of a pioneer tropical tree species: patch dynamics, seed dispersal, and seed banks. Evolution 50(3): 1155-1166.
Borgella R, A.A. Snow, and T.A. Gavin. 2001. Species richness and pollen loads of hummingbirds using forest fragments in southern Costa Rica. Biotropica 33(1): 90-109.
Bruna, E.M. 2003. Are plant populations in fragmented habitats recruitment limited? Tests with an
Amazonian herb. Ecology 84(4): 932-947.
Bullock, J.M. and R.T. Clarke. 2000. Long distance seed dispersal by wind: measuring and modelling the tail of the curve. Oecologia 124(4): 506-521.
Cipollini, M.L., D.A. Wallace-Senft, and D.F. Whigham. 1994. A model of patch dynamics, seed dispersal, and sex ratio in the dioecious shrub Lindera benzoin
(Lauraceae). Journal of Ecology 82: 621-633.
Dean, W.R.J., S.J. Milton, and F. Jeltsch. 1999. Large trees, fertile islands, and birds in arid savanna.
Journal of Arid Environments 41: 61-78.
Dobson, A.P., A.D. Bradshaw, and A.J.M. Baker. 1997. Hopes for the future: restoration ecology and conservation biology. Science 277: 515-522.
Fragoso, J.M.V., K.M. Silvius, and J.A. Correa. 2003. Long-distance seed dispersal by tapirs increases seed survival and aggregates tropical trees. Ecology 84(8):
1998-2006.
Gadgil, M. 1971. Dispersal: population consequences and evolution. Ecology 52: 253-261.
Greene, D.F. and E.A. Johnson. 1995. Long-distance wind dispersal of tree seeds. Canadian Journal of
Botany 73(7): 1036-1045.
Griffith, A.B. and I.N. Forseth. 2002. Primary and secondary seed dispersal of a rare, tidal wetland annual, Aeschynomene virginica . Wetlands 22(4): 696-704.
Harper, J.L. 1977. Population biology of plants . Academic Press, London.
He, H.S., Z. Hao, D.R. Larsen, L. Dai, Y. Hu, and Y. Chang. 2002. A simulation study of landscape scale forest succession in northeastern China. Ecological Modeling
156: 153-166.
______, D.J., Mladenoff, and J. Boeder. 1999. Object-oriented design of LANDIS, a spatially explicit and stochastic forest landscape model . Ecological Modeling 119:
1-19.
Hewitt, N. and M. Kellman. 2002 a . Tree seed dispersal among forest fragments: I. Conifer plantations as seed traps. Journal of Biogeography 29: 337-349.
______ and ______ 2002 b . Tree seed dispersal among forest fragments: II. Dispersal abilities and biogeographical controls. Journal of Biogeography 29: 351-363.
Huxel, G.R. and A. Hastings. 1999. Habitat loss, fragmentation, and restoration. Restoration Ecology
7(3): 309-315.
Jordano, P. and E.W. Schupp. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb . Ecological Monographs 70(4):
591-615.
Levine, J.M. 2003. A patch modeling approach to the community-level consequences of directional dispersal. Ecology 84(5): 1215-1224.
Maina, G.G. and H.F. Howe. 2001. Inherent rarity in community restoration. Conservation Biology 14(5):
1335-1340.
MOBOT (MissOuri BOTanical Garden website). Malagasy/Indo-australo-malesian Phytogeographic
Connections, http://www.mobot.org/MOBOT/Madagasc/phytogeographic/ldd.shtml. Accessed in
December 2003.
Muller-Landau, H.C., S.A. Levin, and J.E. Keymer. 2003. Theoretical perspectives on evolution of longdistance dispersal and the example of specialized pests. Ecology
84(8): 1957-1967.
Nathan, R., G. Perry, J.T. Cronin, A. Strand, and M.L. Cain. 2003. Methods for long-distance dispersal.
Oikos 103: 261-273.
Orth, R.J., J.R. Fishman, M.C. Harwell, and S.R. Marion. 2003. Seed-density effects on germination and initial seedling establishment in eelgrass, Zostera marina , in the
Chesapeake Bay region. Marine Ecology Progress Series 250: 71-79.
Pakeman, R.J., R.F. Pywell, and T.C.E. Wells. 2002. Species spread and persistence: implications for experimental design and habitat re-creation. Applied Vegetation
Science 5: 75-86.
Palmer, M.A., R.F. Ambrose, and N.L. Poff. 1997. Ecological theory and community restoration ecology.
Restoration Ecology 5: 291-300.
Robinson, G.R. and S.N. Handel. 2000. Directing spatial patterns of recruitment during an experimental urban woodland restoration. Ecological Applications 10(1): 174-188.
Ruhren S. and S.N. Handel. 2003. Herbivory constrains survival, reproduction and mutualisms when restoring nine temperate forest herbs. Journal of the Torrey Botanical
Society 130 (1): 34-42.
Tewksbury, J.J., D.J. Levey, N.M. Haddad, S. Sargent, J.L. Orrock, A. Weldon, B.J. Danielson, J.
Brinkeroff, E.I. Damschen, and P. Townsend. 2001. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proceedings of the National
Academy of Science 99(20): 12923-12926.
Turner, M.G., R.H. Gardner, R.V. O’neill. 2001. Landscape ecology in theory and practice: pattern and process . Springer-Verlag, New York.
8
Valverde, T.and J. Silvertown. 1997. An integrated model of demography, patch dynamics, and seed dispersal in a woodland herb, Primula vulgaris . Oikos 80:67-77.
Velland, M. 2003. Habitat loss inhibits recovery of plant diversity as forests regrow. Ecology 84(5):
1158-1164.
Wang, B.C. and T.B. Smith. 2002. Closing the seed dispersal loop. Trends in Ecology and Evolution 17:
379-385.
White, P.S. and S.T.A. Pickett. 1985. Natural disturbance and Patch dynamics: an introduction. In The ecology of natural disturbance and patch dynamics (S.T.A. Pickett and P.S. White, eds.), pp.3-9. Academic Press, Orlando, Florida.
Wiens, J.A. 1989. Spatial scaling in ecology. Functional Ecology 3: 385-397.
Wood, B.C. and A.S. Pullin. 2002. Persistence of species in a fragmented urban landscape: the importance of dispersal ability and habitat availability for grassland butterflies. Biodiversity and Conservation 11(8): 1451-1468.
SOURCE of PHOTOS
Courtesy of Shannon Galbraith : Boone County Cliffs Nature Preserve, Kentucky (first two photos) and Flushing Meadows, Queens, New York City
9






![[CLICK HERE AND TYPE TITLE]](http://s3.studylib.net/store/data/006863514_1-b5a6a5a7ab3f658a62cd69b774b6606c-300x300.png)