Organic Modeling Lab
advertisement

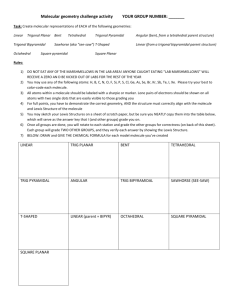
Organic Modeling Lab You will build several 3 carbon organic compounds and determine the following for each a) b) c) d) e) f) The structural formula The electron arrangement about each carbon atom The bond angle between the three carbon atoms the number of bonding pairs and lone pairs in each molecule Presence of polar bonds All intermolecular forces that would be experienced by a large volume of these molecules (make sure to identify which part of the molecule exerts each IMF) To build: 1. Propane 2. Propene 3. Propanal 4. Propanol 5. Propanone 6. Propadiene 7. 2,2-dichloropropane 8. Propanoic acid Organic Modeling Lab You will build several 3 carbon organic compounds and determine the following for each a) b) c) d) e) f) The structural formula The electron arrangement about each carbon atom The bond angle between the three carbon atoms the number of bonding pairs and lone pairs in each molecule Presence of polar bonds All intermolecular forces that would be experienced by a large volume of these molecules (make sure to identify which part of the molecule exerts each IMF) To build: 1. Propane 2. Propene 3. Propanal 4. Propanol 5. Propanone 6. Propadiene 7. 2,2-dichloropropane 8. Propanoic acid Organic Modeling Lab Answers 1) Propane a. b. tetrahedral c. 109.5 d. BP: 10 LP: 0 e. No polar bonds f. Dispersion forces 5) Propanone a. b. tetrahedral, trig planar, tetrahedral c. 120 d. BP: 10 LP: 2 e. C=O f. Dipole-Dipole, Dispersion forces 2) Propene a. b. Tetrahedral, Trig planar, Trig planar c. 120 d. BP: 10 LP: 0 e. No polar bonds f. Dispersion forces 6) Propadiene a. b. trig planar, linear, trig planar c. 180 d. BP: 8 LP: 0 e. No polar bonds f. Dispersion forces 3) Propanal a. b. tetrahedral, tetrahedral, trig planar c. 109.5 d. BP: 10 LP: 2 e. C-O f. Dipole-Dipole, Dispersion forces 7) 2,2-dichloropropane a. b. tetrahedral c. 109.5 d. BP: 10 LP: 6 e. C-Cl maybe f. Dipole-Dipole (maybe) , Dispersion forces 4) Propanol a. b. tetrahedral c. 109.5 d. BP: 11 LP: 2 e. C-O, O-H f. Hydrogen Bonding Dipole-Dipole, Dispersion forces 8) Propanoic Acid a. b. tetrahedral, tetrahedral, trigonal planar c. 109.5 d. BP: 11 LP: 4 e. C-O, O-H f. Hydrogen Bonding, Dipole-Dipole, Dispersion forces