The Respiratory System revised May2008
advertisement

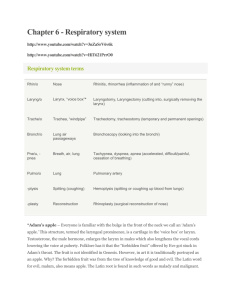
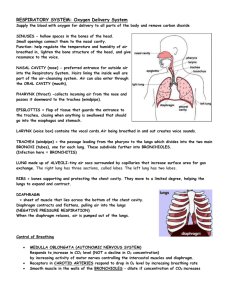
The Respiratory System Grade Level: 5 Standards: 2b. Students know how blood circulates through the heart chambers, lungs, and body and how carbon dioxide (CO2) and oxygen (O2) are exchanged in the lungs and tissues. 6b. Students will develop a testable question. 6c. Students will plan and conduct a simple investigation based on a student-developed question and write instructions others can follow to carry out the procedure.” 6g. Students will record data by using appropriate graphic representations and make inferences based on those data. 6h. Students will draw conclusions from scientific evidence and indicate whether further information is needed to support a specific conclusion. 6i. Students will write a report of an investigation that includes conducting tests, collecting data or examining evidence, and drawing conclusions. Review the following standard: 1g. Students know properties of solid, liquid, and gaseous substances, such as sugar (C6H12O6), water (H2O), helium (He), oxygen (O2), nitrogen (N2), and carbon dioxide (CO2). Objectives: Students build a model of the human lung, and investigate how the lungs work. They make a change to their lung models, and explore how this change affects lung function, and why. Students measure lung capacity, and explore the lung capacity of others in the class, testing for a specific variable of their choice (gender, level of athletic activity, height, etc.). Students analyze their data, draw graphs and write a report on their findings. Background: Do several activities on air pressure before doing this lesson. 1. Air Pressure Activity (Standard 4e): Fill a clear plastic cup partway with water. Place an index card across the top of the cup Ask the class to predict what would happen if I were to turn the cup over and let go of the card. UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 1 of 13 After students write the standard, the hypothesis, the materials and the procedure in their lab report, they go outside to do the activity. Students write their hypothesis using the sentence frame: If______________________, then_____________________________. Students should refer back to the standard for an explanation of what happened. After students have written their conclusion and turned in their lab reports, discuss the results with the class. [The card adheres to the cup and the water doesn’t pour out – this is because the card slightly bulges downward, decreasing the air pressure inside the cup. The outside pressure is slightly higher than the air pressure above the water in the cup and sufficiently higher to keep the card in place.] 2. Air Pressure Demonstration (Standard 4e and 2b) Materials: (Materials in bold are not provided in the kit) 1 clear glass pie dish 1 birthday candle 1 cork (3 cm diameter, not bigger) 1 box of matches 1 500-mL Erlenmeyer flask Water and some food coloring Procedure: 1. Fill pie pan with a ½ inch of water. Add some food color. Any color the students like. 2. Drill a hole partway into the cork and stick the birthday candle inside. 3. Light the candle. 4. Place the cork and burning candle on the desk and ask the students what will happen if you place an inverted Erlenmeyer flask over the candle and the cork? Have students discuss this with their partner and explain their answer. [Students should know that burning a candle requires oxygen and the flame will go out when the oxygen inside the Erlenmeyer flask is used up.] 5. In the pie dish with colored water, place a dime near the middle. 6. Light the candle and place the cork with the burning candle next to the dime; hold onto cork. 7. Ask the students what will happen if you place an inverted Erlenmeyer flask over the candle and the cork in the water? One edge of the Erlenmeyer rests on top of the dime. 8. After students discuss this or after they write a hypothesis, place the Erlenmeyer flask over the candle and the cork in the water. 9. Have students discuss or write down their observations and explain why the water moved up inside of the Erlenmeyer flask. [Oxygen inside the flask was consumed until the candle went out. This results in a lower pressure inside the flask relative to the air pressure outside of the flask. This imbalance in pressure causes the water to be pushed up into the flask until the pressure inside and outside UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 2 of 13 the flask are the same. The dime is used to make it easier for the water to be pushed up into the flask.] Important Point: Air Flows from High Pressure to Low Pressure Lower Air Pressure Higher Air Pressure - pushes water up into the flask Extension: (You may not want to go into this if the students are just learning about pressure). In addition to oxygen being consumed, there is another reason why the pressure inside the flask decreases. The burning candle heats the air inside the flask. As the temperature of a gas increases, the gas expands. You can see some air bubble out of the flask when you first place the flask over the burning candle. After the candle goes out the air inside the flask cools, the gas molecules slow down and the pressure decreases – this decrease in pressure also causes the water to be pushed up into the flask. You may have noticed some condensation inside the flask. After the candle goes out, the cooler air causes the water vapor to condense. Also note that as the candle burns, oxygen gas is consumed and carbon dioxide gas and water vapor is produced. Some of the water does condense and the ratio of oxygen gas consumed is a factor of 1.5 greater than the amount of carbon dioxide produced. Respiratory System Materials: (Materials in bold are not provided in the kit) Materials for pre-activity demonstrations: 1. 2. 3. 4. 5. 6. 7. A pair of safety glasses Potassium Iodide 3% Hydrogen Peroxide 2 votive candles wooden splint (7.5′′ wooden coffee stirrer) 400-mL beaker 500-mL Erlenmeyer flask 8. 9. 10. 11. box of matches salsa cup Baking Soda Vinegar Student Materials Part I 1. 1 small empty water bottle per student (students bring a 500-mL (standard size) water bottle the day before they do the activity) 2. 1 latex glove per student 3. 1 pair of scissors per student (unless you precut the bottles in half) 4. Rubber bands (2 very small (from orthodontist) and 1 medium size per student) 5. One ¼” Sprinkler T-valve per student 6. A 2-inch section ¼” O.D.Tubing (1/8” I.D.) per student UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 3 of 13 Student Materials Part II 1. 2. 3. 4. 5. 6. (Supplies for three stations) Three 1-gallon milk jugs, or 2-Liter soda bottles 3 plastic or rubber hoses (2-3 feet long and 1/4" diameter) 3 large containers (dish-pan or some other plastic container to hold water) 1 straw per student 400 mL beaker, or measuring cup notepad and pencil to record data VOCABULARY:1 Lungs: Teach vocabulary before students do the activity. Lungs are organs in the human body essential to respiration which bring O2 into your body and into the bloodstream, and get rid of waste CO2 produced by cells. Oxygen: Oxygen is a chemical element which has the symbol “O” in the periodic table. At room temperature and pressure, oxygen exists as a diatomic molecule with the formula O2, in which two oxygen atoms are bonded to each other. O2 is a gas molecule that makes up ~20% of the air and is essential to human life. Carbon Dioxide: Carbon dioxide is a chemical compound made of one carbon atom and two oxygen atoms. The molecular formula for carbon dioxide is CO2. CO2 is a gas at room temperature and pressure. We exhale CO2. Respiration: Cellular respiration is the process by which an organism obtains energy from the reaction of glucose and oxygen to form water and carbon dioxide. We inhale oxygen and exhale carbon dioxide. Trachea: The trachea is a large tube, sometimes called the “windpipe” through which air passes on its way to the lungs. Bronchial trees: (or Bronchi) the branches following the trachea before the lungs, which resemble two upside-down trees. Alveoli: Alveoli are tiny, microscopic sacs at the end of the bronchial trees where O2 and CO2 gases are exchanged. Diaphragm: The major muscle used in breathing, located beneath the lungs. Lung Capacity: The maximum volume of air the lungs can hold. Air Pressure: The force exerted on us and on everything around us by tiny particles of air (air molecules). UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 4 of 13 Introduction - LUNGS 1. Introduce lungs as an important organ in the human body. Show students where the lungs are located - within your chest cavity inside the rib cage. 2. Ask students – where are your lungs? Which lung is your left lung? Which lung is your right lung? Have the students discuss this with their partner for a minute, have them point to their own left and right lungs, and those of their partner. 3. What are the lungs used for? Ask the students to take a deep breath. What happens to this area of your body? (point to the chest and rib area) It expands and contracts—how can the lungs do this? They are made of spongy, elastic tissue that expands and contracts as you breathe. The airways that bring air into the lungs (the trachea and bronchi) are made of smooth muscle and cartilage, allowing the airways to contract and expand as well. The lungs and airways bring in fresh, oxygen-enriched air and get rid of waste carbon dioxide made by your cells. (Show overhead and go over vocabulary on the overhead) Diagram: (overhead provided) UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 5 of 13 Pre-Activity Demonstrations(and infomation):3 Carbon dioxide, CO2, and oxygen gas, O2 We eat carbohydrates (starches) and fats to produce energy which is used to maintain our body temperature, to do work by contracting muscles and to construct and repair tissues in our body. We can break down starches in the intestines to produce glucose, C6H12O6. Glucose dissolves in the blood and is known as “blood sugar.” When we breathe in, we inhale vital oxygen gas. When we exhale, we breathe out waste carbon dioxide gas produced by your cells. This is basically the result of respiration, in which glucose reacts with oxygen to produce carbon dioxide, water and energy. C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy Energy CH2OH H C C HH OH C OH O H C OH H + C OH Work + Heat O + 6 6 O=O 6 O=C=O oxygen, O2 carbon dioxide, CO2 H H water, H2O Glucose: C6H12O6 Draw the structure of glucose on the board. Have students count all the carbon atoms, oxygen atoms and hydrogen atoms in glucose to show that the molecular formula for glucose, C6H12O6, means there are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Distinguishing between gases: Both oxygen and carbon dioxide are clear, colorless gases. However, they have very different properties. To show the differences between these two gases, perform the following steps in a demonstration to the class. Carbon Dioxide Gas Demonstration (practice doing the demonstration before you do this for your students): 1. Light a votive candle, and ask the class to discuss what they think fire is with their partners. Fire is a result of the release of heat from a chemical reaction called combustion (commonly known as burning) and light due to electronic transitions within molecules. What do we need to start a fire? (fuel, oxygen and a spark, or heat to start the reaction) What do you think will happen if we take away the oxygen? (have the students discuss this with their partner) How can we remove O2? Have students discuss their ideas to remove O2. 2. Some students may suggest placing a container over the candle. You can use the Erlenmeyer flask to do this. As the candle burns, oxygen is used up and students can see the flame flicker and slowly go out. Is there another way to remove the oxygen gas? UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 6 of 13 3. Place a salsa cup, upside-down in a 400-mL beaker. Sprinkle about a quarter teaspoon of baking soda around the salsa cup in the beaker. Place a lit votive candle on the salsa cup. Pour in about two teaspoons of vinegar, just enough to cover the bottom of the beaker. Gently swirl the beaker. Observe the reaction. 4. What happened? Ask the students to discuss with their partner what happened and why they think it happened. Walk around with the beaker so the students can see the bubbling taking place from the reaction. The candle will go out because CO2 is produced, which displaces the O2, and therefore extinguishes the flame. The following reaction is taking place: CH3COOH acetic acid + NaHCO3 sodium bicarbonate NaCH3COO + H2CO3 sodium acetate carbonic acid Acetic acid (vinegar is ~3% acetic acid in water) plus sodium bicarbonate (baking soda) makes sodium acetate plus carbonic acid. The H2CO3 (carbonic acid) then breaks down into water and carbon dioxide gas: H2CO3 H2O + CO2 5. Try to relight the candle. It won’t light. Why not? We need oxygen gas to relight the candle. How do we get rid of the carbon dioxide gas? Can we pour it out? 6. To emphasize this idea, light a second votive candle and place it inside a beaker. Carefully “pour” the carbon dioxide gas on the flame of the second candle without spilling the remaining vinegar. The flame goes out. Ask the students to discuss why this happened. CO2 is denser than air, therefore it sinks, and puts out the candle. Fire extinguishers use CO2 to put out fires for this reason! 7. Now that we poured out the CO2 from the first beaker, can we relight the candle? Relight the candle to show that this works. Oxygen Gas Demonstration: 1. In the Erlenmeyer flask, pour ~ 100 mL of hydrogen peroxide, H2O2. Add a teaspoon of potassium iodide, KI, and hold up the flask so the students can observe what is happening. What are the bubbles? Allow this reaction to run for a full minute. 2. Light the end of a 7.5′′ wooden coffee stirrer and allow it to burn until it is glowing red. Blow out the fire and quickly place it in the Erlenmeyer flask. It should relight. If it does not, allow more reaction time, or make sure you use finely granulated potassium iodide instead of large chunks. You can blow out the stick and relight it many times, until the oxygen is used up. You can also extinguish the flame in the beaker containing CO2 gas. Ask the students to discuss why they think this happened with their partner. The chemical reaction that occurred is: KI 2 H2O2 2 H2O + O2 Note that potassium iodide, KI, is written above the arrow. KI acts as a catalyst to speed up the reaction but is not consumed in the overall reaction. Oxygen gas is produced, allowing UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 7 of 13 the candle to relight. Our bodies use oxygen in a complex process to digest the food that we eat into useful energy for our bodies. When you don’t eat for a long time, you feel fatigued because your body is not producing enough energy to function properly. 3. There is one main reason why we can breathe. It is because “Air flows from high pressure to low pressure.” Have the students repeat this phrase then do the demo on this topic. Air Flows from High Pressure to Low Pressure Air pressure is the force exerted on you by tiny particles of air (air molecules). The molecules exert a force on you because they are moving very fast, and bouncing off of you with a very high frequency (if you multiply a million times a million times a million times a million, that’s the number of collisions that take place per second per square inch!) How much pressure are you under? Earth's atmosphere is pressing against each square inch of you with a force of 1 kilogram per square centimeter (14.7 pounds per square inch). The force on 1,000 square centimeters (a little larger than a square foot) is about a ton! Why doesn't all that pressure squash me? Remember that you have air inside your body too, that air balances out the pressure outside so you stay nice and firm and you are not squashed. Balloon (or bicycle pump) Demonstration: Blow up the balloon. Ask the students what gas molecules are inside the balloon? What is happening inside the balloon to keep it inflated? The molecules inside the balloon are moving very fast (~1000 miles/hour at room temperature) and they collide with each other and with the inside walls of the balloon. The pressure inside the balloon is a little higher than the pressure outside the balloon due to the force exerted by the balloon on the gas molecules inside the balloon. What happens if you pop or release the balloon? (do this—you can do both or either, depending on how your neighboring colleagues feel about loud noises) Air rushes out of the balloon, from an area of higher pressure to an area of lower pressure. You can also bring in a bicycle pump. Cover up the air outlet of the pump, as you press down the handle, the volume inside the pump decreases and the pressure increases. When you remove your hand from to outlet air rushes out. Part I: Construction of the lungs (~1 hour) 1. Students bring in a 500-mL (standard size) water bottle one day prior to the activity for preparation. First, using a drill, drill a ¼′′ hole in the cap of the water bottle. (it’s easier to do this if you leave the cap on the bottle) If you don’t have a drill, a sharp pointed pair of scissors will work to make a a ¼′′ hole or a piece of latex can be used in place of the cap. In this case, have students cut a piece of latex glove, fix it over the mouth of the bottle with a rubber band and poke a hole in the top. 3. Use a box cutter to puncture a hole in the bottle about half-way down. This will allow the students to cut the bottle in half with scissors (if you are short on class time you can precut the bottles using the box cutter for the students). 4. Give the remaining instructions to the class as a whole, and hand out one latex glove, one T-valve, 2 small rubber bands, one larger rubber band, and one short section of clear tubing. They should all have a bottle with a cap with a hole, or with the alternative latex cap. UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 8 of 13 Lung Model Push diaphragm up (lungs deflate) Pull diaphragm down (lungs inflate) 4. Take a small piece (~2”) of the clear rubber tubing, and push one end over the top of the T-valve. 5. Cut the fingers off of a latex glove. 6. Take one of the latex fingers and bunch together the open end and place the rubber band twice around the end. Push the open end of the finger onto one side of the T-valve and role the rubber band over the T-valve. This is the most difficult part of this activity. Do this again with a second latex finger on the other side of the T-valve. These are the lungs. (see diagram) 7. Take the cap on the bottle and drop the lungs into the bottle so that the tubing protrudes out the neck of the bottle. Push the end of the tube through the cap. Replace the cap on the bottle. The T-valve is upside-down in the bottle, with the lungs hanging down. 8. Next cut a piece of latex from the palm of a glove large enough to cover the bottom of the bottle. This will be the diaphragm. 9. Use a rubber band (twice around) to secure to diaphragm to the bottom of the bottle. You have made your lungs! 10. As shown in the picture of the lung model, use one hand to keep the rubber band and diaphragm in place. With the other hand gently pull down on the latex diaphragm and then push it up. What happens to the lungs? Have the students discuss this with their partners, and discuss why this happens. UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 9 of 13 Why do the lungs behave this way??? When we inhale, the diaphragm and intercostal muscles (those are the muscles between your ribs) contract and expand the chest cavity. This expansion lowers the pressure in the chest cavity below the outside air pressure. Air then flows in through the airways (from high pressure to low pressure) and inflates the lungs. When you exhale, the diaphragm and intercostal muscles relax and the chest cavity gets smaller. The decrease in volume of the cavity increases the pressure in the chest cavity above the outside air pressure. Air from the lungs (higher pressure) then flows out of the airways to the outside air (lower pressure). The cycle then repeats with each breath. The lung model operates in the same way. As you pull down on the latex diaphragm, the volume inside the plastic bottle (chest cavity) increases, which results in a decrease in pressure. The molecules inside the bottle now have more room to move around in and therefore collide less frequently with the walls of the cavity and lungs. As a result, air rushes into the lungs, inflating them. When the diaphragm is pushed up, the volume in the chest cavity decreases, so the pressure in the cavity increases. The added pressure on the outside surface of the lungs increases, pushing out the air inside the lungs. Review Questions: As we inhale the diaphragm moves downward – how does this change the volume inside the lungs? If the volume increases, what happens to the pressure? What causes air to rush in when as we inhale? All students say: Air moves from high pressure to low pressure. The changes in volume and pressure in the lungs allows us to breathe. At this point, move to the lung construction, Procedure, Part I, step 6. Procedure: Part II. Measuring Lung Capacity4 (~1 hour) There are three different measurements of lung capacity that can be done. Vital capacity is the largest amount of air that can be exhaled after taking a deep breath. Tidal volume is the amount of air taken in during normal breathing. Expiratory reserve is the air that is left in the lungs after exhaling in normal breathing. Hypothesis: Before students do this activity, ask them to come up with some factors they think might affect one’s vital capacity. Have them vote on their favorite (i.e. height, gender, physical activity, etc.). For an average adult, the amount of air that can be exhaled from a deep breath is about 4.5 liters (8.4 pints). A well-trained athlete has a greater lung capacity. The average amount of air he or she could exhale from a deep breath is 6.5 liters (12 pints). In the second part of this experiment, your students will measure their lung capacity. UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 10 of 13 Diagram: 1. Begin by pouring 250 mL aliquots of water into an empty 3 liter bottle using a beaker. Mark the water line on the bottle with a permanent marking pen. Add 250 mL more, mark the water line. Continue until container is filled with water. Empty the bottle and turn it right side up. From the bottom to the top, at each water line write the volume, 250 mL, 500 mL, 750 mL, 1000 mL….using the permanent ink marker. 2. Fill the dishpan about 1/4 full of water. Keep in mind the bucket or dishpan must be deep enough to accommodate the added height of an inverted soda bottle or gallon jug. 3. Hold your hand tightly over the mouth of the jug and turn the jug upside down in the dishpan. Remove your hand when the mouth of the bottle is below the water level line. The point here is to minimize any air bubbles in the bottle. 4. Put a clean straw into one end of the plastic hose and place the other end into the mouth of the jug (about 10 cm or 4 in). Keep the hose pinched or air may leak into the jug and water may leak out. A student will need to hold the bottle so that it doesn't fall over. 5. To get a measure of the vital capacity, have the students exhale a deep breath into the hose. To prepare for this activity have the students take a deep breath and then exhale normally. Encourage the students not to exhale the remainder of air in their lungs, this may cause dizziness. 6. To measure the volume read the amount of water displaced using the marks on the bottle. Record the students name and the volume exhaled. 7. To set-up easily for the next student, use a funnel to carefully pour water from the tub back into the emptied 2 liter bottle. Now you're ready for the next person! 8. After the students have done this activity, ask them to discuss what factors did affect vital lung capacity. They need to organize the class data and graph their data. For example, they can graph height versus volume. NOTE: You may prefer to do the lung capacity measurement activity before students build the lung model. Either way will work fine. UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 11 of 13 Diversity/ESL: Have students work individually, but with a discussion partner. Try to pair together students who work well together, and whenever possible, pair an English learner with a student who is speaks English fluently. Throughout the lesson students discuss the answers to questions with their partner before sharing their answers with the class. Do the record and play back activity5 to reinforce the concepts learned, and to help English learners develop language proficiency by carefully listening to pronunciation and by repeating what they heard (see recorder activity in the language section below). Extensions/Integrations: Art: Students can take their lung models home, or to art class, and add arms, legs, a head, etc. to resemble a human body. Math: Lung capacities will be averaged, and results presented in graphical form. Language: 1. Students give an oral presentation on the lung capacity experiment that they performed. 2. Students write up their results in a report on the lung model and the lung capacity measurements. The report should include a discussion of building the lung model and how the model is used to demonstrate breathing. In their report, students should use and underline all the vocabulary words given at the start of this activity. They should also discuss the scientific concepts needed to understand breathing and how oxygen and carbon dioxide gases are exchanged in the lungs. Let students know that they will receive points for each of the vocabulary words and for each scientific concept that they use in their report. 3. The following day have students do a record and play back activity to reinforce the concepts learned, and to help English learners develop language proficiency by carefully listening to pronunciation and by repeating what they heard. 4. Students are paired up, an English learner with a student who is fluent in English, and given a prompt to discuss some aspect of the lungs, breathing or the carbon dioxide and oxygen gas demonstrations. a. Using the prompt, one student tells another student anything that he knows about this topic for about a minute. The other student listens carefully and after the minute is over repeats back as much as possible of what he/she heard. b. The roles of speaker/recorder are switched. Give a different prompt for discussion. c. Select students to share their discussion with the class. Example Prompts: 1. 2. 3. 4. 5. 6. Describe respiration. What gases are consumed and produced in this process? In cellular respiration energy is produced. What reaction occurs to produce this energy and what is the energy used for? Discuss breathing and the changes in pressure that occur as we breathe. Discuss the lung model and how it demonstrates breathing. Carbon dioxide and oxygen are colorless invisible gases at room temperature and pressure. What experiment can we do to distinguish between these two gases? How did you measure lung capacity? What factors affect lung capacity? UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 12 of 13 Assessment: Have the students answer the questions on the work sheet in class. Safety: There are no hazardous materials used in these activities. Safety glasses must be worn while you perform the CO2 and O2 demonstrations. Be sure to warn the students if you plan on popping the balloon as some students are easily startled. Also, be sure that the students use their own straw for the lung capacity experiments to avoid the spread of germs. Resources: (1) (2) (3) (4) (5) http://en.wikipedia.org/wiki/Main_Page Info adapted from: http://health.howstuffworks.com/lung1.htm Activity and info adapted from: http://kids.earth.nasa.gov/archive/air_pressure/ Activity and info adapted from: http://www.caosclub.org/freelessons/hbody3.html Recorder Activity: Craig Strang, UC Berkeley, cstrang@berkeley.edu Contact Information: Website: Petra van Koppen, Ph.D. Department of Chemistry and Biochemistry vankoppen@chem.ucsb.edu http://outreach.chem.ucsb.edu Fifth grade teachers measuring their lung capacity (above) and building the lung model (left). The Materials and Chemistry Outreach Program is supported by: The Camille and Henry Dreyfus Foundation, UCSB Faculty Outreach Grants, The Department of Chemistry and Biochemistry UCSB Chemistry Outreach Program. Lauren Benz and Petra Van Koppen Page 13 of 13