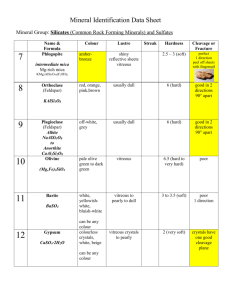
Felsic Silicon to Oxygen ratio: (1:2) Name comes from “feldspars
advertisement

Felsic Silicon to Oxygen ratio: (1:2) Name comes from “feldspars” Felsic minerals cover most of Earth’s surface Light colored: pink, white, yellow Less dense Lower melting temperature: easy to melt, hard to crystallize Ultramafic/Mafic Silicon to Oxygen ratio: (1:4) Name comes from “magnesium and iron (Latin: ferrum)” Make up most of mantle Dark colored: black, green Denser Higher melting temperature Quartz Formula: SiO2 Most felsic mineral Has lots of forms, many of which don’t look alike Physically and chemically durable; more chemically durable than diamonds 7 on Mohs Scale of Hardness; is the hardest common mineral, as only gemstones are harder Gemstone- attractive minerals used to make jewelry Heavily represented in sedimentary rocks because everything else gets broken down 99.9% of quartz is NOT in crystalline form In the real world, contaminants, temperature changes, etc. keep crystals from forming in ideal shape Crystal face- flat side Termination- point at crystal’s end Cleavage- tendency of mineral to break along flat surfaces Quartz has no cleavage; instead, it has conchoidal fracture, a curved breakage with ripple patterns that also occurs in obsidian Quartz crystals are hexagonal (6 crystal faces) Some varieties of quartz: Milky- grayish Crystal- mineral grains distinctive; transparent Rose- pink Citrine- orange, crystalline Amethyst- purple Agate- has layered bands Jasper- darker agate Chalcedony- rose shapes Chert- forms on seafloor Smoky- structural damage by radiation causes color Tigerseye- orange with optical effect Aventurine- green Feldspars Taken collectively, most common minerals in crust are feldspars Less felsic than quartz Not as durable as quartz with only medium chemical durability Hardness of 6 on Mohs Scale Feldspars at Earth’s surface decay into clay from chemical weathering over time Orthoclase (K-spar)- potassium feldspar; cleavages intersect at 90° Plagioclase- Sodium feldspar and Calcium feldspar 3 types of potassium feldspar: orthoclase, sanidine, microcline Can use color to distinguish orthoclase and plagioclase 90% of the time Orthoclase- pink to orange Plagioclase- white Labradorite- pretty, iridescent plagioclase Can sometimes be mixed up with quartz Apart from color, cleavage is the best way to tell them apart Micas Group of minerals with hardness of about 3 or 4 on Mohs Scale Generally flat, sheeted ,flaky, and shiny minerals that can be peeled with fingernails (part of the Mica experience) Has one prominent cleavage Called sheet silicates because of the sheet-like arrangement of its atoms Often found in streambeds (ends up on top of sediments because it’s light) and in sand Was used for windows because it’s naturally flat and regular; also used for its dielectric strength 3 common types : Muscovite (name after Moscow, where it was mined)- light, silvery color; little more felsic than biotite. Formula: KAl2(AlSi3)O10(OH)2 Biotite- black to brown color; little more mafic than Muscovite. Formula: K(Mg, Fe)3(Al, Fe)Si3O10(OH, F)2 Lepidolite- purple color; has structure and chemical content similar to those of muscovite (sometimes same mineral grain is silver, then purple, then silver); the main ore of lithium; an indicator of gemstones Quartz, feldspars, and micas make up the bulk of felsic, igneous rocks (like granite) Amphiboles/Pyroxenes- both big, complicated mineral groups. Most common amphibole is Hornblende Most common pyroxene is Augite Hornblende Black, shiny, lustrous Amphibole: less dense with evident cleavage planes at 60°/120° Hardness of 6 Felsic Augite Has some similar properties to hornblende: black, shiny, lustrous Pyroxene: denser with cleavages meeting at 90° Hardness of 6 Mafic: therefore, it occurs in different rocks from hornblende Olivine Most mafic mineral (Mg, Fe)2SiO4 Green to yellow color Translucent Vitreous (glassy) texture no cleavage Weathers rapidly at earth’s surface into oxides and serpentine Most of mantle and lower crust is olivine; found in basalt Iron olivine is greener, magnesium olivine is yellower Peridot- gem-quality olivine Dunite- rock that’s 90% olivine Miscellaneous Rock Facts Making rocks wet brings out color and contrast Polishing a rock’s surface makes this wet-effect permanent Cooling something gradually forms few nucleation sites, allowing a small number of crystals to grow; cooling something fast creates several nucleation sites, leaving lots of small crystals That’s why liquid nitrogen ice cream takes so much creamier (smaller crystals) Earth’s radius is 4,000 miles (6,380 km) Zoning- subtle changes in chemical composition in different rock layers Mass spectrometer- device that identifies the chemical composition of a compound or sample based on the mass-to-charge ratio of charged particles; the ratio is calculated by passing the particles through electric and magnetic fields Polarizing microscope- used to identify rocks and minerals in thin sections; optical properties of the minerals in the thin section alter the color and intensity of the light as seen by the viewer Olivine and feldspar seen under a polarizing microscope: Non-silicates- minerals not made up of silicon and oxygen Calcite CaCO3 Almost always biogenic (formed by organisms); lots of organisms build calcite shells Seashells and coral reefs are made of calcite Plankton have shells of calcite Coquina- made of shells stuck together Majority of world’s carbon found in calcite (more than that in living things, air, fossil fuels, and ocean combined) Test for calcite: apply hydrochloric acid to mineral, and it will bubble CaCO3 + 2HCL CO2 + H2O + CaCl2 Marble, limestone, chalk, and travertine are all made up of calcite Limestone- sedimentary rock that’s mostly calcite (generally from shells); most common sedimentary rock not made of debris; main ingredient in concrete Marble- metamorphic product of squeezing and heating limestone (intense pressure and heat) Chalk- made of microscopic pieces of calcite shed from micro-organisms Travertine- calcite formed in layers, often in found hot springs Hematite Rust (Fe2O3) Distinctly darkly pigmented (dark red/brown rust color) Usually in the form of a microscopic powder and a trace component in rocks Gives rocks a reddish hue Mars is red because of the hematite on its surface Hematite is the end result of chemical weathering of iron (oftentimes found in mafic rocks) Main ore of iron Streak- color of the powder produced when it a mineral is dragged across an unweathered surface Ceramic tiles are often used as streak plates for streak tests Hematite has a distinctive dark red streak, even though solid hematite is black BIF- banded-iron formation; sedimentary rocks with structures consisting of repeated thin layers of iron oxides, either magnetite or hematite, alternating with bands of iron-poor shale and chert Soil in wet places has hematite Hematite: Magnetite Fe3O4 (Fe3+, Fe3+, Fe2+ with O2-) Most common and strongest of all naturally magnetic minerals Magnetite grains are positioned along Earth’s magnetic fields “lodestone”- naturally magnetized magnetite that was used as an early compass Made when lightning strikes magnetite; very rare) Can create artificial magnetic magnetite by heating it up and cooling it down quickly in a magnetic field Powder is black; leaves a black streak Similar to hematite: exists as microscopic powder in other rocks You can distinguish between hematite and magnetite by using a streak test or a magnet Turns into hematite after being subject to weathering on earth’s surface Hardness- how difficult it is to scratch something; different from strength Diamond is hard, but very brittle (will shatter if hit with hammer) Mohs Hardness Scale 10- Diamond 9- Corundum 8- Topaz 7- Quartz 6- Orthoclase feldspar 5- Apatite 4- Fluorite 3- Calcite 2- Gypsum 1- Talc Mnemonic: The Gay Cowboy Found An Old Quaker To Corundum Diamond Talc Softest sort of common mineral Whitish, greenish, grayish color Is an amphibole Slippery texture Used in talcum (baby) powder Can carve it up with fingernails (hardness of 2.5) Gypsum Calcium sulfate: CaSO4 or CaSO4 • 5H2O (hydrate- holes in crystal structure that can trap water) Is an evaporite- forms when water evaporates (like in a desert’s dry lake beds) Looks like calcite Very soft: can be scratched with fingernails; usually powdery Sometimes crystallizes, as in selenite Used to make plaster, like in drywall Gypsum: Selenite: Diamond Just carbon (C), like charcoal Can only form naturally 10 km below Earth’s surface; probably not rare in the mantle Not stable at earth’s surface: slowly turning into graphite Only found in “kimberlites”- weird blocks of rock raised quickly from the mantle Very shiny: has highest known index of refraction in a naturally occurring mineral Index influences how much light bends in mineral Also influences the critical angle, angle of incidence, above which all light is reflected and bounces We have the means to make large synthetic, gem-quality diamonds at cheap prices, but the De Beers Group is against it Industrial diamonds are produced for stuff like heat sinks Corundum 2nd hardest naturally occurring mineral Al2O3 Generic name that includes the colorful, transparent gemstones: ruby and sapphire Rubies- red (colored by chromium) Sapphires- color other than red; can be cooked to become blue/bluer Synthetic corundum used to make watch faces (scratch-proof) and knives Anything with hardness 8 or above is pretty much scratch-proof since it cannot be scratched by sand (quartz)