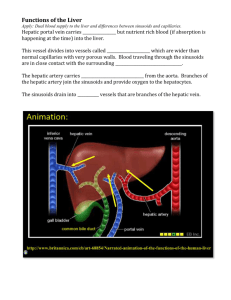
Supplemental online Materials and Methods:
advertisement

Supplemental online Materials and Methods: Immunohistochemical detection and MetaMorph quantitation of cells expressing beta galactosidase Four millimeter slices of tissue were fixed overnight in formalin-zinc, rinsed in PBS, embedded in paraffin and sectioned every 5 microns. Sections were deparaffinized by successive washes in Hemo-D, 100%, 95%, 70%, and 50% ethanol, double distilled water, and PBS. Endogenous peroxide activity was eliminated with a 3% solution of hydrogen peroxide in methanol, followed by rehydration in water. Sections were blocked in 5% goat serum in PBS (Jackson ImmunoResearch, West Grove, PA). The sections were incubated with mouse anti-beta-galactosidase (0.1 µg/ml, Roche, Cat # 1083104) overnight at 4oC, washed twice in PBS, followed by incubation with affinity purified, peroxidase labeled goat anti-mouse IgG (1.2 µg/ml, Jackson ImmunoResearch, Cat # 115-035-062) for one hour at 37oC, followed by two washes in PBS and one wash in 0.5 mM Tris-HCl pH 7.5. Peroxidase label was detected using the Liquid DAB SubstrateChromogen System (DAKO, Cat # K3466) per the manufacturer’s instructions. Sections were then counterstained with Methyl Green. Metamorph (Universal Imaging Corporation, Downingtown, PA) software was used to identify and quantitate the nuclei of hepatocytes and non-hepatocytes infected with Ad2-gal. Color development times for immunohistochemical detection of betagalactosidase signal were held constant to ensure consistent MetaMorph detection of expressing cells. Each liver section was scanned into MetaMorph, and 30 to 45 one mm 2 regions were chosen from each liver section for MetaMorph analysis. Color thresholding was used to distinguish the brown DAB signal representing positive beta-galactosidase nuclei from the blue-green nuclei of non-infected cells. Color thresholding and all subsequent MetaMorph classifications were optimized by preliminary empirical analysis of the nuclei from a sample liver section, followed by human validation of each classification. To classify nuclei into hepatocytes, non-hepatocytes or unknown objects, the “total area” and “elliptical form factor” of all beta-galactosidase positive nuclei were measured by MetaMorph. The total area (TA) was defined as the sum all the contiguous pixels of a given -galactosidase positive nucleus that met the color threshold. The ellipitical form factor (EFF) was defined as the length of a given beta-galactosidase positive nucleus divided by its breadth. For example, the EFF of a perfect circle is 1.0, while the EFF of an ellipse is generally greater than 1.25. The nuclei of single hepatocytes are roughly spherical and were characterized by an EFF≤1.25 and a TA=16 to 18 pixels. The nuclei of double hepatocytes appear as two closely spaced spheres that histologically cannot be dissected by MetaMorph, and produced an EFF that was generally greater than 1.5, with a TA>18 pixels. The betagalactosidase positive nuclei of non-hepatocytes are predominantly derived from the liver macrophages (Kupffer cells) and endothelial cells, and are characterized by an EFF>1.25 and a TA >6 and ≤18 pixels. Due to the position and orientation of a given nucleus within the sectional plane, some beta-galactosidase positive nuclei were classified as unknown objects because they could not be unequivocally classified into hepatocytes or non-hepatocytes. Unknown objects were assumed to represent hepatocytes and non-hepatocytes equally, and were not factored into any quantitative analysis. Unknown objects had a TA equal to or greater than 1 but less or equal to 6 pixels and any value for EFF, or a TA greater than 6 but less than 16 pixels and an EFF ≤ 1.25. Five 1mm2 fields in proximal, medial and distal sections from each of the four main lobes of the rabbit liver were analyzed by MetaMorph analysis. To assess background readings from our MetaMorph analysis we performed beta-galactosidase staining and MetaMorph analysis of liver sections from one naïve and one passively immunized rabbit that were subjected to sham injection of saline. MetaMorph detected few very small artifacts as well as a small number of hepatocytes and non-hepatocytes. However, the artifacts were below our previously established lower limit of TA (6 contiguous pixels) of what constitutes a true nucleus, and the average number of hepatocytes and non-hepatocytes detected among five separate fields were 1.6 ± 3.1 and 3.4 ± 4.6, respectively for naïve shams, and 1.3 ± 1.7 and 3.3 ± 3.7, respectively, for passively immunized shams. These values were deemed negligible and were ignored in our final MetaMorph assessments.