- Immune Tolerance Network
advertisement
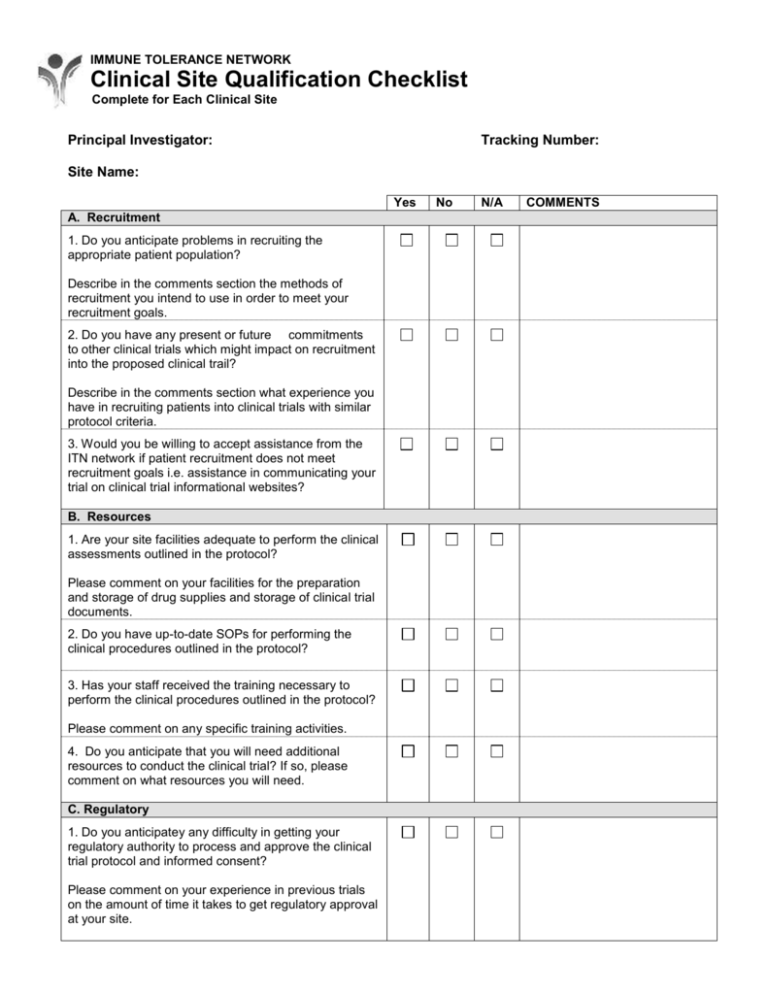
IMMUNE TOLERANCE NETWORK Clinical Site Qualification Checklist Complete for Each Clinical Site Principal Investigator: Tracking Number: Site Name: Yes A. Recruitment 1. Do you anticipate problems in recruiting the appropriate patient population? Describe in the comments section the methods of recruitment you intend to use in order to meet your recruitment goals. 2. Do you have any present or future commitments to other clinical trials which might impact on recruitment into the proposed clinical trail? Describe in the comments section what experience you have in recruiting patients into clinical trials with similar protocol criteria. 3. Would you be willing to accept assistance from the ITN network if patient recruitment does not meet recruitment goals i.e. assistance in communicating your trial on clinical trial informational websites? B. Resources 1. Are your site facilities adequate to perform the clinical assessments outlined in the protocol? Please comment on your facilities for the preparation and storage of drug supplies and storage of clinical trial documents. 2. Do you have up-to-date SOPs for performing the clinical procedures outlined in the protocol? 3. Has your staff received the training necessary to perform the clinical procedures outlined in the protocol? Please comment on any specific training activities. 4. Do you anticipate that you will need additional resources to conduct the clinical trial? If so, please comment on what resources you will need. C. Regulatory 1. Do you anticipatey any difficulty in getting your regulatory authority to process and approve the clinical trial protocol and informed consent? Please comment on your experience in previous trials on the amount of time it takes to get regulatory approval at your site. No N/A COMMENTS Immune Tolerance Network Site Qualification Checklist Page 2/2