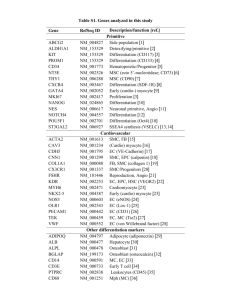
Use of endothelial progenitor cells differentiated from human
advertisement
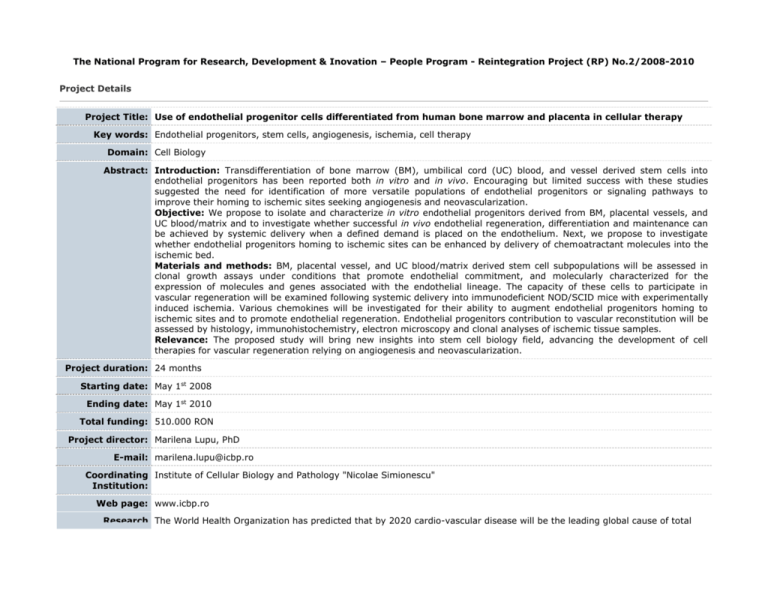
The National Program for Research, Development & Inovation – People Program - Reintegration Project (RP) No.2/2008-2010 Project Details Project Title: Use of endothelial progenitor cells differentiated from human bone marrow and placenta in cellular therapy Key words: Endothelial progenitors, stem cells, angiogenesis, ischemia, cell therapy Domain: Cell Biology Abstract: Introduction: Transdifferentiation of bone marrow (BM), umbilical cord (UC) blood, and vessel derived stem cells into endothelial progenitors has been reported both in vitro and in vivo. Encouraging but limited success with these studies suggested the need for identification of more versatile populations of endothelial progenitors or signaling pathways to improve their homing to ischemic sites seeking angiogenesis and neovascularization. Objective: We propose to isolate and characterize in vitro endothelial progenitors derived from BM, placental vessels, and UC blood/matrix and to investigate whether successful in vivo endothelial regeneration, differentiation and maintenance can be achieved by systemic delivery when a defined demand is placed on the endothelium. Next, we propose to investigate whether endothelial progenitors homing to ischemic sites can be enhanced by delivery of chemoatractant molecules into the ischemic bed. Materials and methods: BM, placental vessel, and UC blood/matrix derived stem cell subpopulations will be assessed in clonal growth assays under conditions that promote endothelial commitment, and molecularly characterized for the expression of molecules and genes associated with the endothelial lineage. The capacity of these cells to participate in vascular regeneration will be examined following systemic delivery into immunodeficient NOD/SCID mice with experimentally induced ischemia. Various chemokines will be investigated for their ability to augment endothelial progenitors homing to ischemic sites and to promote endothelial regeneration. Endothelial progenitors contribution to vascular reconstitution will be assessed by histology, immunohistochemistry, electron microscopy and clonal analyses of ischemic tissue samples. Relevance: The proposed study will bring new insights into stem cell biology field, advancing the development of cell therapies for vascular regeneration relying on angiogenesis and neovascularization. Project duration: 24 months Starting date: May 1st 2008 Ending date: May 1st 2010 Total funding: 510.000 RON Project director: Marilena Lupu, PhD E-mail: marilena.lupu@icbp.ro Coordinating Institute of Cellular Biology and Pathology "Nicolae Simionescu" Institution: Web page: www.icbp.ro Research The World Health Organization has predicted that by 2020 cardio-vascular disease will be the leading global cause of total background: disease burden. One out of every ten people who have a myocardial infarction dies within a year. Although neoangiogenesis within the infarcted bed is an integral component of the remodelling process, the capillary network is unable to support the great demands of the hypertrophied myocardium, resulting in progressive loss of viable tissue, infarct extension and fibrous replacement1. Here we propose to use selected populations of endothelial progenitors to induce new blood vessel formation in the infarcted bed (vasculogenesis) and proliferation of the pre-existing vasculature (angiogenesis) after experimental vascular ischemia, as well as to investigate efficient ways to improve endothelial progenitors homing to ischemic sites. A large body of evidence indicates that small populations of pluripotent stem cells exist in many tissues, including bone marrow (BM), umbilical cord (UC) blood (UCB), and blood vessels. During development, endothelial cells are derived from mesoderm. Commitment of the hemangioblast to the endothelial lineage is characterized by sequential expression of vascular endothelial (VE)-Cadherin (CD144), PECAM-1 (CD31), and shortly afterward CD342. In postnatal life, endothelial progenitors express AC133, Flk1, CD34, and MUC183-5, markers that are quickly lost upon differentiation to mature endothelium. Von Willebrand factor (vWF), VE-Cadherin, Weibel-Palade bodies, PECAM-1, and endoglin (CD105) are markers expressed by mature endothelial cells6. BM-derived cells have been shown to possess the potential to transdifferentiate into endothelial cells 7 and induce therapeutic angiogenesis of ischemic tissues8-12. Multipotent adult progenitor CD34-, VE-Cadherin-, AC133+, and FLk1+13 cells have been identified in the non-hematopoietic BM compartment; when cultured with VEGF, these cells differentiate into CD34 +, VECadherin+, AC133-, and FLk1+ cells, a phenotype characteristic for angioblasts, and subsequently differentiate into cells that express endothelial markers and contribute to neoangiogenesis in vivo13. Endothelial progenitors have also been isolated from human peripheral blood3;9;10;14. Human BM stem cell compartments are highly complex and perhaps comprising other cell types whose endothelial fate we propose to define. Vessel-associated progenitor cells, so called pericyte derived cells (in post-natal life) or mesangioblasts (during embryonic development)15;16, are common progenitors for vascular and extravascular mesodermal derivatives. They express early endothelial markers such as stem cell antigen-1(Sca-1), Flk-1, CD34, MEF2D, kit and VE-Cadherin15. Mesangioblasts have been isolated from embryonic mouse aorta15 and the pericyte derived cells have been isolated from vessels of juvenile dystrophic mice17, outgrowth of small vessel-containing tissue fragments from muscle of adult dogs18, and micro-vasculature within the BM19. When systemically injected, both mesangioblasts and pericyte derived cells were able to accumulate in the first capillary filter encountered, transit through the endothelial barrier only in the presence of inflammation15;16, and improve muscle function both in dystrophic dogs and mice17;18. We propose to investigate whether pericyte derived cells could be isolated from human placental vessels and contribute to angiogenesis and neovascularization. The UC is composed of connective tissue called Wharton Jelly, covered by a simple epithelium believed to derive from amniotic membrane. It has been shown that UC epithelium has the ability to undergo terminal differentiation when grafted onto the back of nude mice20. Our rationale to use UC matrix as a source of endothelial progenitors is based on the fact that several groups postulated that the UC matrix is a rich source of self-renewing, multiple-lineage differentiating cells21;22; however, these cells have not been fully characterized. A few groups addressed the use of UCB stem cells to generate endothelial progenitors23-28. We propose to identify and further characterize human UC blood/matrix stem cells with ability to differentiate towards endothelial lineage and participate to vascular regeneration. Elevated serum levels of chemokines such as stromal-derived factor-1 (SDF-1), vascular endothelial growth factor (VEGF), and angiopoietin-1 were able to induce mobilization of endothelial progenitors29. SDF-1 is produced within the bone marrow and mediates chemokinesis and chemotaxis on a variety of cell types that express the CXCR4 receptor. SDF-1-responsive cell types include CD34+ hematopoietic progenitors and stem cells. High mobility group box 1 (HMGB1), a chromatin protein that acts as a cytokine when released in the extracellular milieu by the necrotic and inflammatory cells, and its receptor for advanced glycation end products (RAGE) were shown to induce both migration and proliferation of vessel-associated stem cells30;31. Therefore we plan to investigate whether local delivery of such chemotactic molecules within the ischemic sites is able to improve/accelerate vascular repair and regeneration. Research Stem cell plasticity studies have a huge potential for both basic and translational research. There is compelling evidence from relevance: both in vitro and in vivo experiments that BM and UCB derived stem cells can contribute to endothelial regeneration. This has led to the proposal that humans with serious vascular injuries can potentially benefit from cellular therapies using the above and perhaps other cell types such as placental vessel and UC matrix derived stem cells. The proposal seeks to demonstrate the capacity of human BM, placental vessel, and UC blood/matrix derived endothelial progenitors to contribute to angiogenesis and neovascularization and to gain insight into the biology of undifferentiated progenitor cells. The selection, characterization, and improved delivery of autologous stem cell populations endowed with tissue specific plasticity appears to be the obvious next step towards applying stem cell therapy as a regenerative therapy. We intend to follow this avenue of research with the goal of developing a feasible therapeutic approach for human patients with vascular diseases. The regenerative stem cell therapy represents an innovative, less invasive and less expensive treatment strategy that could address a large spectrum of chronic degenerative disorders. Both morbidity and mortality associated with these disorders have severe socio-economic consequences. Within the medical community, studies resembling those proposed here raise hopes for the improvement of population general health status. The proposed research has a highly significant potential for immediate clinical translation “from bench to bedside”. Systemic and repeated delivery of autologous endothelial progenitors for the therapeutic management of vascular diseases would be a simple and safe procedure in human patients. Furthermore, endothelial progenitor cells isolated at birth from the placental tissues can be stored and used, as needed, for autologous cell therapy purposes. Another potential advantage of the use of placental-derived stem cells for cell therapy is that they are less immunogenic and are associated with much lower rates of graft-versus-host disease (GVHD) after allogeneic transplantation compared to adult stem cells; therefore, placental-derived stem cells could be a safer source for tissue-targeted allogeneic cell therapy than BM-derived stem cells. Data protection: Project results will be posted after their publication in scientific journals. 1. Colucci WS. Molecular and cellular mechanisms of myocardial failure. Am.J.Cardiol. 1997;80:15L-25L. Bibliography: 2. Nishikawa SI, Nishikawa S, Kawamoto H et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761-769. 3. Peichev M, Naiyer AJ, Pereira D et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000;95:952-958. 4. Gehling UM, Ergun S, Schumacher U et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000;95:3106-3112. 5. Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J.Clin.Invest 2000;105:71-77. 6. Woywodt A, Haubitz M, Buchholz S, Hertenstein B. Counting the cost: markers of endothelial damage in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:1015-1023. 7. Shi Q, Rafii S, Wu MH et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998;92:362-367. 8. Bhattacharya V, McSweeney PA, Shi Q et al. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood 2000;95:581-585. 9. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-967. 10. Takahashi T, Kalka C, Masuda H et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat.Med. 1999;5:434-438. 11. Folkman J. Therapeutic angiogenesis in ischemic limbs. Circulation 1998;97:1108-1110. 12. Kalka C, Masuda H, Takahashi T et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc.Natl.Acad.Sci.U.S.A 2000;97:3422-3427. 13. Reyes M, Dudek A, Jahagirdar B et al. Origin of endothelial progenitors in human postnatal bone marrow. J.Clin.Invest 2002;109:337-346. 14. Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J.Clin.Invest 2000;106:571-578. 15. Minasi MG, Riminucci M, De AL et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 2002;129:2773-2783. 16. Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr.Opin.Genet.Dev. 2003;13:537-542. 17. Sampaolesi M, Torrente Y, Innocenzi A et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intraarterial delivery of mesoangioblasts. Science 2003;301:487-492. 18. Sampaolesi M, Blot S, D'Antona G et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006;444:574-579. 19. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001;19:180-192. 20. Sanmano B, Mizoguchi M, Suga Y, Ikeda S, Ogawa H. Engraftment of umbilical cord epithelial cells in athymic mice: in an attempt to improve reconstructed skin equivalents used as epithelial composite. J.Dermatol.Sci. 2005;37:2939. 21. Hoynowski SM, Fry MM, Gardner BM et al. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem.Biophys.Res.Commun. 2007;362:347-353. 22. Can A, Karahuseyinoglu S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of FetusDerived Stem Cells. Stem Cells 2007 23. Wu X, Lensch MW, Wylie-Sears J, Daley GQ, Bischoff J. Hemogenic Endothelial Progenitors Cells Isolated from Human Umbilical Cord Blood. Stem Cells 2007 24. Schmidt D, Asmis LM, Odermatt B et al. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann.Thorac.Surg. 2006;82:1465-1471. 25. Chiu B, Wan JZ, Abley D, Akabutu J. Induction of vascular endothelial phenotype and cellular proliferation from human cord blood stem cells cultured in simulated microgravity. Acta Astronaut. 2005;56:918-922. 26. Droetto S, Viale A, Primo L et al. Vasculogenic potential of long term repopulating cord blood progenitors. FASEB J. 2004;18:1273-1275. 27. Murohara T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc.Med. 2001;11:303-307. 28. Crisa L, Cirulli V, Smith KA et al. Human cord blood progenitors sustain thymic T-cell development and a novel form of angiogenesis. Blood 1999;94:3928-3940. 29. Moore MA, Hattori K, Heissig B et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann.N.Y.Acad.Sci. 2001;938:36-45. 30. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191-195. 31. Palumbo R, Sampaolesi M, De MF et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J.Cell Biol. 2004;164:441-449.