Biology 6B
advertisement

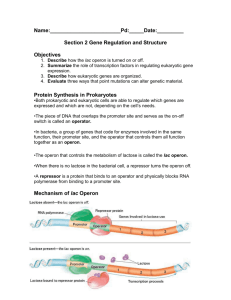
3-7 Biology 22 Operons REGULATION OF GENE EXPRESSION IN PROKARYOTES Follow diagrams in your textbook and notes from the lecture to construct each of the operon models described below. A. CONSTITUTIVE SYSTEMS (e.g. general metabolic enzymes) 1. Construct a model system: Use yellow pop beads to represent 4 structural genes. Use red pop beads to represent the mRNA transcribed from these structural genes. Use blue pop beads to represent the protein enzymes translated from the mRNA. Use 11 red pop cylinders to represent the promoter. Use the wooden block labeled "RNA polymerase" to represent that enzyme. 2. Set up the model and run it through all steps: Have the polymerase attach to the promoter, then pass along the structural genes to produce mRNA. Have the mRNA separate from the DNA and use it to synthesize proteins. 3. Consider what would happen if each of the following were to occur: a mutation removed half of the promoter region. one base was substituted in the middle (-25) of the promoter region. five bases were added to the middle of the promoter region. one nucleotide was substituted at position –35. the start codon was deleted from the first structural gene. 3-8 B. The Lactose Operon: An Inducible Operon under both Negative and Positive Control The product of the regulatory gene I is an active repressor that binds to the operator in the absence of lactose. When lactose binds to the repressor, the repressor can no longer block the path of RNA polymerase from the promoter to the structural genes. Positive control is exerted by the cAMP + CAP complex. CAP is produced in an inactive form. When glucose levels are low, cAMP builds up. CAP is activated by cAMP binding and the complex attaches to the promoter to enhance transcription. 1. Construct a model of the operon: Use yellow pop beads to represent the three structural genes: lacZ, lacY, lacA. Use red beads to represent the mRNA and blue beads to represent the protein gene products. Use 9 blue pop cylinders to represent the operator. Use 6 red pop cylinders to represent one portion of the promoter and one red pop cylinder with a white glass rod to represent the promoter region called CBS (CAP binding site) where the CAP + cAMP complex attaches. Use the tubing labeled cAMP to attach to the tubing labeled CAP. In order to enhance RNA polymerase binding, cAMP + CAP must bind to the CBS region of the promoter. Remember that cAMP and glucose concentrations are inversely proportional. Use 8 yellow pop cylinders for the regulatory gene (I) and red pop beads for its mRNA. Use the wooden block labeled "active repressor protein" for the regulatory gene product. Note how the lactose block can be used to alter its shape. 2. Show how the operon works under the following conditions: a. High lactose and high glucose in the environment. b. High lactose and low glucose in the environment. c. Low lactose and high glucose in the environment. d. Low lactose and low glucose in the environment. 3-9 B. The Lactose Operon (continued) 3. Consider the effects of mutations in each of the regions of this operon: the promoter for the structural genes is deleted. the operator region is deleted. a mutation in the lacI gene such that the gene product can no longer bind to lactose. the start codon for the lacZ gene is deleted. a mutation in CAP such that it can bind to cAMP but the complex can no longer bind to the promoter for the lac structural genes. 4. If a fourth structural gene were inserted after the third one by genetic engineering techniques, what cellular conditions would lead to its being expressed? Would the identify of the gene make any difference (i.e. whether or not it were related to lactose in its activity)? Would it make any difference, if it were a eukaryotic gene such as insulin, whether the introns had been removed from the gene? 3-10 C. The Arabinose Operon: Inducible Operon under both Positive and Negative Control For the arabinose operon, the same protein, the product of the araC gene, exerts both negative and positive control. The araC product is a negative regulator (active repressor) when arabinose is not bound to it. AraC binds to both araI and araO creating a looped DNA structure that prevents RNA polymerase from attaching to the promoter. AraC is a positive regulator (enhancing transcription) when it binds to arabinose. The arabinose + araC product complex binds to the araI region containing the promoter for the araB, araA and araD structural genes to stimulate transcription. This operon is also under the positive control of the cAMP + CAP complex. Transcription of the structural genes is optimized when both the arabinose + araC and cAMP + CAP complexes are attached to their binding sites on the common promoter for araB, araA and araD. Transcription of the structural genes is rare in the absence of these positive regulators. 1. Construct a model of the operon: Use yellow pop beads to represent the three structural genes: araB, araA and araD. Use red pop beads for mRNA, and blue pop beads for proteins. Use 6 red pop cylinders and one red pop cylinder with a white glass rod to represent the promoter region called CBS (CAP binding site) where the CAP + cAMP complex attaches. Use 6 blue pop cylinders to represent the operator and 6 additional blue pop cylinders to represent the I site on the promoter where araC + arabinose bind. Use 8 yellow pop cylinders to represent the regulatory gene, araC. Use red pop beads for the mRNA of the regulatory gene. Use the wooden block labeled "control protein" to represent the gene product of the regulator gene. Note that the arabinose block can change the shape of the control protein. 2. Show how the operon works under the following conditions: a. High arabinose and high glucose in the environment. b. High arabinose and low glucose in the environment. c. Low arabinose and high glucose in the environment. d. Low arabinose and low glucose in the environment. 3-11 3. Consider the effects of mutations on each part of this system: (in all cases, assume the environment is high in arabinose and low in glucose) deletion of the I region. deletion of the O region. mutation in the araC gene such that the product cannot bind to arabinose. mutation in the araC gene such that the product can bind to arabinose but the araC + arabinose complex cannot bind to the I region. mutation in the CAP gene such that the product cannot bind to cAMP. mutation of the I region so that it can no longer bind the arabinose + ara C complex. 3-12 3-13 D. TRYPTOPHAN OPERON: A REPRESSIBLE OPERON WITH ATTENUATION The tryptophan operon contains an "attenuator" region in the leader sequence between the operator and the first structural gene. The attenuator is transcribed by RNA polymerase, but if tryptophan is present, it interacts with the new transcript fragment and causes the polymerase to fall off the DNA and transcription to stop. If the polymerase gets beyond the attenuator region and into the first structural gene, the transcript fragment gets folded in such a way that tryptophan can no longer interact with it. 1. Construct a model system: Use yellow pop beads to make five structural genes: trpE, trpD, trpC, trpB, trp A. Use red and blue pop beads for mRNA and proteins. Use 9 blue pop cylinders to represent the operator. Use 6 red pop cylinders to represent one portion of the promoter. Attach 6 white pop cylinders to represent the L region containing the attenuator. Use 8 yellow pop cylinders to represent the regulatory gene. Use red pop beads to represent the mRNA of the regulatory gene. Use the wooden block labeled "repressor protein" to represent the product of the regulatory gene. Use the small wooden block labeled "co-repressor" to represent tryptophan. Note how this block can be used to alter the shape of the "repressor protein" block. The repressor protein is active only when it is bound to tryptophan. Describe the conditions under which tryptophan can regulate the rate of transcription of this operon. 3-14 2. Use the model to illustrate how this operon carries out transcription and translation under the following conditions: High tryptophan in the environment. Low tryptophan in the environment together with rapid protein synthesis in the cell (uses tryptophan up). Rapid synthesis of tryptophan with a low rate of protein synthesis in the cell (does not use tryptophan at a rapid rate). How many "start transcription" signals are there in this operon? How many "stop transcription" signals? How many signals are there to start and stop translation? 3. Consider events that could disrupt this operon such as: What mutations in the operator could make this operon constitutive? Could shut it off permanently? What mutations in the promoter could alter the rate of transcription? How serious would each one probably be? How would mutations in the regulator gene affect the operon? How would mutations in the structural genes affect the operon? Which mutations would potentially have the greatest effect on the ability of the bacterium to survive? 4. Tryptophan levels in the cell are also controlled by feedback inhibition, where tryptophan inhibits the activity of the enzyme encoded by the trpE and trpD genes. When tryptophan levels build up in the cell, which type of control would be exerted first, feedback inhibition or repression of tryptophan operon transcription? Be sure that you can explain your answer. 3-15