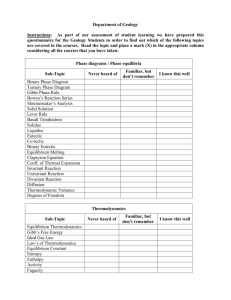
ERSC 4016: IGNEOUS AND METAMORPHIC PETROLOGY
advertisement

ERSC 4016: IGNEOUS AND METAMORPHIC PETROLOGY OUTLINE 2004-2005 Pre-requisites: ERSC 3301 Mineralogy II Instructor: Sobhi Nasir, Room D418 (sobhi@squ.edu.om) Lectures: Saturday, Monday, 11:00 a.m., Room 0042N Labs: Wensday 2:00 to 4:00; Room 0042N Introduction Igneous rocks: nomenclature and classification; magmatic processes. Application of one, two and three component phase diagrams to interpret the formation of igneous rocks. Metamorphic rocks: types, classification, mineral parageneses. Labs: the study of rocks in thin section, their mineralogy, textures, origin and classification. Recommended Text: Best - Igneous and Metamorphic Petrology Yardly – Metamorphic petrology Supplementary Texts - Available in the Main Library Petrography and Petrology Blatt and Harvey - Petrology, Igneous, Sedimentary and Metamorphic Cox, Bell and Pankhurst - The Interpretation of Igneous Rocks Hatch, Wells and Wells - Petrology of the Igneous Rocks Mason - Petrology of the Metamorphic Rocks Griffiths - Petrology of the Sedimentary Rocks Moorehouse - The Study of Rocks in Thin Section Williams, Turner and Gilbert - Petrography Sood - Modern Igneous Petrology Spry - Metamorphic Textures Winkler - Petrogenesis of Metamorphic Rocks Carmichael, Turner and Verhoogen - Igneous Petrology Evaluation First Test: 10% Midterm 20% Final 35% Labs 25% Assignments (3) 10% OBJECTIVES 1. Introduction and application of Phase chemistry to igneous rocks relate the direct results of experimental work to the study of natural processes. 2. Develop a working knowledge of common mineral associations in classes or groups of igneous and metamorphic rocks. Concentrated in Laboratories 3. Introduction to igneous rock forming processes. An attempt to predict where igneous rocks originate and how they evolve by post melting processes = differentiation. 4. Introduction to Metamorphic Rocks Classification, lithologic types Lecture Outline Igneous Rocks Definition Generalities Nomenclature and Classification Silica Gradation/Colour Gradation/Saturation Concept Chemistry Modal Analysis Normative Analysis Variation Diagrams Fractionation Liquid Immiscibility Hybridization and Assimiliation Fractional Crystallization Phase Chemistry and Phase Diagrams Introduction Unary Systems Phase Rule Binary diagrams Introduction Definitions Getting Started Phase Rule Lever Rule Crystallization in Binary Systems Binary System #1 - Simple system (Di-An) Equilibium Crystallization Fractional Crystallization Melting Binary System # 2 - Congruently Melting Intermediate Compound Binary System # 3 - No Solid Solution, Incongruently Melting Compound (Fo-Qtz) Equilibrium Melting Equilibrium Crystallization Fractional Crystallization Binary System # 4 - Solid Solution (Ab-An) Equilibrium Crystallization Fractional Crystallization Binary System #5 - Ab-Or Ternary Systems Introduction Phase Rule Compositions Hypothetical Ternary Systems Rules Directions of Falling Temperature Isothermal Sections Ternary System #1 - Simple Ternary System (An-Wo-Sp) Equilibrium Crystallization Fractional Crystallization Ternary System #2 - Incongruent Melting (Fo-Si-An) Equilibrium Crystallization 1 Equilibrium Crystallization 2 Fractional Crystallization Ternary System # 3 - Solid Solution (Di-Ab-An) Composition, Proportions, Tie Lines Equilibrium Crystallization 1 Equilibriium Crystallization 2 Fractional Crystallization Metamorphism Introduction Temperature limits Terms Metamorphic Facies Types of Metamorphism Compositional Groups Barrovian Metamorphism o Introduction o Barrow's Zones o Interpretation DEFINITION and VARIABILITY IGNEOUS ROCKS Solidified from molten material or a MAGMA MAGMA - naturally occurring liquid (mobile rock material) generated within the earth, capable of intrusion and/or extrusion from which igneous rocks have been derived by solidification and other processes. Magma may or may not contain: Solids - crystals, xenocrysts, xenoliths or rock fragments Gases - dissolved gaseous phases in the liquid LIQUID PHASE: Chemical species in liquid phase consist of metallic ions, e.g. Fe2+ (ferrous), Fe3+ (ferric), Mg2+, Na+ etc. held within a discontinuous, fluctuating matrix of variously linked Si, Al and O atoms. The relative abundance of the ions present is dependant on the composition of the magma and the physical conditions, T and P, under which the magma exists. GAS PHASE: Most melts are capable of containing gases dissolved in solution in the liquid phase Gas species include H2O, CO2, H2S, CH4 (Methane), NH3 (Ammonia) With a decrease in Pressure, gases come out of solution and form discrete gas bubbles in the liquid - produces vesicles in volcanic rocks, responsible for explosive nature of volcanic eruptions. The composition of the gas phase varies widely in magmas reflecting the composition, T and P of formation, source material etc. SOLID PHASE: May be crystals which form early as the result of crystallization from the liquid or rock fragments, xenoliths, or inclusions of country rocks. VARIABILITY Igneous rocks are not everywhere abundant on and/or within the earth. They generally lack large scale mineral deposits, so they have very minor economic significance. Igneous activity, volcanism, provides the only samples available with which we may directly study the composition of the Earth's interior. Material samples the lower crust and upper mantle, the outer 100 km of the earth. Igneous rocks are extremely variable, mineralogically and chemically, yet a link between the original and final liquid composition can often be inferred. Two possibilities exist to explain the variety of igneous rock types observed: 1. all rocks were originally created differently, or 2. some process(s) exist which has/have the ability to generate a variety of rock types from a universal, original starting composition. The latter possibility is only applicable to a restricted or particular locality. Source compositions are sufficiently different with each composition undergoing similar process(s) after derivation. These processes which can effectively change the composition and character of the magma are termed fractionation or differentiation mechanisms. Which are defined as: formation of variety of liquids of variable composition from an initially homogeneous, single parental material. Will look at fractionation latter in the term. GENERALITIES CONCERNING MAGMAS Cooling 1. magmas do not crystallize suddenly, crystallization occurs over a temperature interval of approx. 100-600°C, more commonly 100-200°C. The rate of cooling depends on the depth, shape of chamber, crystallizing phases, etc. 2. Magmas cool exothermically, releasing heat into the host rock or atmosphere. 3. Magmas are open systems, which allows for the transfer of material, dissolved gases and fluids with the surroundings. 4. Cooling is influenced by the environment. Magmatic Temperatures 1. Extruded magmas = volcanics, are emplaced at atmospheric conditions, not influenced by Pressure. The composition of the magma affects the crystallization temperature. For Basaltic rocks the observed temperature range varies from 9001500°C, commonly the range is 1000-1200°C. 2. Intruded Magmas = plutonic rocks, are emplaced at depth within the crust, and as sych Pressure effects the temperature of crystallization. With increasing pressure the temperature of crystallization decreases. Granitic plutons crystallize at approx. 600°C. Rates of Ascent and Cooling These are related to the structure and physical characteristics of the magma body, e.g. size, shape, viscosity, composition, etc. Ascent- upper limit as inferred for kimberlites, which rise from the mantle at 40 km/hour. - lower limit, a few cm/1000 years Cooling, ranges from quenched material extruded at the surface to plutonic bodies. A medium to large batholith with a volume of 10,000 km3 may require up to 10 million years to completely cool NOMENCLATURE AND CLASSIFICATION Numerous means exist for classifying and naming rocks, all of which arise because as humans we feel we have to recognize and categorize common or contrasting features in related things. Systems of nomenclature and classification may reflect: genetic, textural, chemical or mineralogical features. 1. GENETIC basic system which classifies rocks on the basis of where they form. plutonic - at depth hypabyssal - intermediate depth volcanic - on the Earth's surface. This system is not very practical, but it serves as a first approximation, it tells nothing about mineralogy, chemistry of the rocks and can not distinguish basalt from rhyolite. 2. TEXTURAL relies on the grain size of individual minerals in the rock. aphanitic - fine grained < 1 mm phaneritic - medium grained 1 to 5 mm coarse grained (pegmatitic) > 5 mm This system has the same shortcomings as a genetic classification, however specific textures present may aid in classification, e.g., phenocryst, ophitic, coronas, but these are not indicative of a specific environment of formation or a specific lithology. 3. CHEMICAL This type of classification requires a complete chemical analysis of the rock in order to pigeonhole a sample, and is not practical under field conditions where only a hand lens and hammer are available. A chemical classification system has been proposed for volcanic rocks and a comparable scheme for plutonic rocks is not available. This leaves us with a system based on mineralogy. 4. MINERALOGICAL The one gaining application is the result of several years work by the IUGS Subcommission on the Classification of Igneous Rocks or Streckeissen Classification. IUGS CLASSIFICATION SYSTEM This is based on Modal Mineralogy (MODE - an accurate representation of the distribution and volume percent of minerals within a given rock). The system is simple to use, can be applied in the field, using a hand lens and a hammer. It is based on the percentages of Q (quartz), A (alkali feldspar), P (plagioclase), and F (feldspathoids, e.g. nepheline, leucite). Further subdivisions are dependant on the type and percentage of mafic minerals present. Two systems have been proposed - Handouts. 1. Plutonic Rocks - widely used with universal application o QAPF Quadrilateral o Gabbro Classification 2. Volcanic Rocks - not as popular. HISTORICAL CLASSIFICATION SYSTEMS Several aspects which historically have played and continue to play a role in the classification of igneous rocks should also be considered. 1. GRADATION IN SILICA CONTENT - referred to as acid or basic, implying a range of silica content. o Acidic > 66 wt% SiO2 Granites ~ 72 wt% SiO2, granodiorites ~ 68 wt% SiO2 o Intermediate - 52 to 66 wt% SiO2 Andesite 57 wt% SiO2 o Basic - 45 to 52 Wt% SiO2 Basalts range from 48 to 50 wt% o Ultrabasic - < 45 wt% SiO2 peridotites 41 to 42 wt% SiO2 2. COLOUR GRADATION o o Felsic rocks are light coloured, contain felsic minerals (e.g. qtz, feldspar, feldspathoids) which are themselves light in colour and have a low density which contribute to the pale colour of the rock. Mafic Rocks are denser and dark coloured, the result of containing mafic minerals (pyroxene, amphibole, olivine, biotite). These minerals contribute to the green, brown and black colour of these rocks. Ultramafic vs. ultrabasic 3. SATURATION CONCEPT Used in reference to the SiO2 and Al2O3 which are the two most abundant components of igneous rocks. SiO2 Saturation HANDOUT SiO2 Saturation Minerals present in igneous rocks can be divided into two groups: 1. Those which are compatible with quartz or primary SiO2 mineral (tridymite, cristobalite) these minerals are saturated with respect to Si, e.g feldspars, pyroxenes. 2. Those which never occur with a primary silica mineral. These are undersaturated minerals, e.g. Mg-rich olivine, nepheline. The occurrence of quartz with an undersaturated mineral causes a reaction between the two minerals to form a saturated mineral. 2SiO2 + NaAlSiO4 ===> NaAlSi3O8 Qtz + Ne ===> Albite SiO2 + Mg2SiO4 ===> 2MgSiO3 Qtz + Ol ===> En Rock Classification (Silica saturation) 1. Oversaturated - contains primary silica mineral 2. Saturated - contains neither quartz nor an unsaturated mineral 3. Unsaturated - contains unsaturated minerals Al2O3 Saturation HANDOUT Al2O3 Saturation Four subdivisions of rocks independant of silica saturation, based on the molecular proportions of Al2O3, Na2O, K2O and CaO applied mainly to granitic lithologies. 1. 2. 3. 4. Peraluminous - Al2O2 > (Na2O + K2O + CaO) Metaluminous - Al2O3 < (Na2O + K2O + CaO) but Al2O3 > (Na2O + K2O) Subaluminous - Al2O3 = (Na2O + K2O) Peralkaline - Al2O3 < (Na2O + K2O) Silica Saturation Incompatible Phases Under magmatic conditions some minerals react with free silica to form other (more silica-rich) minerals. These reactant minerals are said to be undersaturated (with respect to SiO2). Other minerals are stable (can coexist) with free silica (generally in the form of quartz) and are said to be saturated (with respect to SiO2). Typical reactions are: 2SiO2 + NaAlSiO4 =======> NaAlSi3O8 quartz + nepheline =======> albite 2SiO2 + KAlSiO4 =======> KAlSi3O8 quartz + kalsilite =======> orthoclase SiO2 + KAlSiO4 =======> KAlSi2O6 quartz + kalsilite =======> leucite SiO2 + Mg2SiO4 =======> 2MgSiO3 quartz + Mg-rich olivine =======> enstatite Shand (1927) proposed the following list of minerals, subdivided on the basis of silica saturation and/or undersaturation, i.e. those that coexist with quartz (+Q) and those that do not coexist with quartz (-Q). Saturated (+Q) Undersaturated (-Q) all feldspars leucite all pyroxenes nepheline all amphiboles sodalite micas cancrinite fayalite (Fe-rich olivine) analcite spessartine Mn3Al2(SiO4)3 forsterite (Mg-rich olivine) almandine Fe3Al2(SiO4)3 melanite (Ti garnet) sphene andradite zircon Ca3(Fe,Ti)2(SiO4)3 topaz pyrope Mg3Al2(SiO4)3 magnetite perovskite ilmenite melilite apatite corundum calcite Undersaturated and saturated minerals can coexist stably under magmatic conditions, but quartz, tridymite and christobalite can only coexist stably with saturated minerals. For example Q + ne is an impossible igneous assemblage, as is Q + ol (Mg - rich) (see reactions above), but Q + ol (Ferich) is stable. Al2O3 Saturation Alumina Saturation Independant of the silica saturation, alumina saturation is based on the 1:1 alkali:alumina ratio of feldspars and feldspathoids. Any excess or deficiency in alumina in a rock is reflected in the mineralogy. Four classes of alumina saturation/undersaturation are: 1. Peraluminous - Al2O3 > (CaO + N2O + K2O) corundum appears in the norm Minerals present in the rock are: muscovite, topaz, tourmaline, spessartine-almandine, corundum, andalusite and sillimanite. 2. Metaluminous - Al2O3 < (CaO + Na2O + K2O) but Al2O3 > (Na2O + K2O). anorthite appears in the norm Al-bearing minerals are typical, e.g. biotite, hornblende. 3. Subaluminous - Al2O3 = (Na2O + K2O) normative anorthite is small Feldspars and feldspathoids are the only minerals with essential Al2O3. 4. Peralkaline - Al2O3 < (Na2O + K2O) and rarely Al2O3 < K2O Acmite, sodium silicate and rarely potassium silicate appear in the norm. Alkali ferromagnesium minerals common, e.g. aegerine, riebeckite, richerite CHEMISTRY OF IGNEOUS ROCKS The chemical composition of rocks is determined by analyzing a powder of the rock. This aspect of geology is carried out in ERSC 3P31 - Geochemistry. Routine geochemical analysis of geologic materials can be carried out using either or a combination of the following two techiques: 1. X-ray Fluoresence Spectroscopy (XRF) to determine both major and trace elements 2. Atomic Absorbtion Spectrometry (AAS) to determine both major and trace elements Specialized techniques and or equipment are necessary to determine other elements. The composition of an igneous rock is dependant on: 1. 2. 3. 4. Composition of the source material Depth of melting Tectonic environment where crystallization occurs. e.g. rifting vs. subduction Secondary alteration Generally three groups of elements are analysed in Igneous rocks. Elements Analyzed in Petrogenetic Studies 1. Major Elements These are the 13 major oxide components which are reported as weight percent (wt%). Major Elements as Oxides Range in OXIDE Normal Igneous Rocks SiO2 35 - 80 wt% Al2O3 8 - 22 wt% TiO2, Fe2O3 (ferric), FeO (ferrous), MnO, MgO,CaO 4 - 30 + Wt% Na2O 1.5 - 8 + wt% K2O 0.5 - 8 + wt% H2O+,- Varies P2O5 < 0.15 wt% CO2 Varies 2. Because these are reported as a percentage the total should sum to 100 %, ideally, however acceptable totals lie in the range 98.5 to 101 wt%. 3. Appendix 1 in the text gives the average chemical composition for a variety of Igneous rocks. 4. Minor or Trace Elements Values for these elements fall in the ppm range and are rarely reported in terms of wt %. Elements include: Li, Be, Sc, V, Cr, Co, Ni, Cu, Zn, Ga Rb, Sr, Y, Zr, Nb Ba, Pb plus F, Cl, S. Theoretically any element can be analyzed for, but you must be aware of the overall composition of the rock, e.g.: Basalt - analyze for Cr, Ni, Cu, but not Li, Be, Ba as these are not important or neccassary to analyze for as they are not present in detectable amounts. Granite pegmatite with lepidolite mica, analyze for Li, Be, and Ba but not Cr, Ni, Cu. 5. Rare Earth Elements (REE or lathanides atomic number 57 to 71), are reported in ppm or mg/g. The REE are important for petrogenetic studies, because as a group the REE behave coherently. Whenever you see a published whole rock or mineral analysis ask if it is a good analysis, Does the total add to 100%. MODAL ANALYSIS Two types of analysis are useful when examining Igneous Rocks: 1. Modal analysis - requires only a thin section, 2. Normative analysis - requires a chemical analysis. MODAL ANALYSIS Produces an accurate representation of the distribution and volume percent of the mineral within a thin section. Three methods of analysis are used: 1. Measure the surface area of mineral grains of the same mineral, relative to the total surface area of the thin section. 2. Measure the intercepts of each mineral along a series of lines. 3. POINT COUNT - Count each mineral occurrence along a series of traverse line across a given thin section. For a statistically valid result > 2000 individual points must be counted. The number of grains counted, the spacing between points and successive traverse lines is dependant on the mean grain size of the sample. Advantages 1. One can compare rocks from different areas if you only have a thin section, no chemical analysis is required, using a petrographic microscope. 2. Gives the maximum and minimum grain sizes. Disadvantages 1. Meaningless if the sample has a preferred orientation of one or more minerals. 2. Porphyritic rocks are difficult to count. 3. Total area of sample must be sufficiently larger than the max. diameter of the smallest grain size NORMATIVE ANALYSIS OR NORM Normative analysis is defined as the calculation of a theoretical assemblage of standard minerals for a rock based, on the whole rock chemical composition as determined by analytical techniques. The original purpose for the norm was essentially taxonomic. An elaborate classification scheme based on the normative mineral percentages was proposed. The classification groups together rocks of similar bulk composition irrespective of their mineralogy. Various types of NORMs have been proposed - CIPW, Niggli, Barth. Each of theses proposals has its own specific advantages and/or disadvantages. The CIPW norm, originally proposed in 1919, was proposed as a means of comparing and classifying all igneosu rocks for which chemical analyses wers available. The NORM takes it's name from the four authors who proposed it Cross, Iddings, Pirsson and Washington. This NORM was very elegant and based on a number of simplifications: 1. The magma crystallizes under anhydrous conditions so that no hydrous minerals (hornblende, biotite) are formed. 2. The feromagnesium minerals are assumed to be free of Al2O3. 3. The Fe/Mg ratio for all feromagnesium minerals is assumed to be the same. 4. Several minerals are assumed to be incompatible, thus nepheline and/or olivine never appear with quartz in the norm. Since the CIPW NORM was introduced in 1919 several other normative calculations have been suggested, e.g. Niggli norm, Barth mesonorm. The latter is used commonly when examining granitic rocks. VARIATION DIAGRAMS COMPOSITIONAL VARIATIONS A main objective of any research program on igneous rocks is to describe and display chemical variations for simplicity and to facilitate condensing information. The best way to simplify and condense analytical data is by graphical means. Harker Diagrams The oldest method is the variation diagram or Harker diagram which dates from 1909, and plots oxides of elements against SiO2. HANDOUT Within province chemical variation The table presented at the top half of the handout presents chemical data for a suite of volcanic rocks, from a single volcano, located in the Red Sea area. The results are listed, from 1 to 13, by increasing SiO2 content - with the actual SiO2 contents varying from 45.5 wt % to 61.22 wt%. With increasing Silica the following trends are evident: 1. TiO2, FeO, MgO and CaO decrease in abundance 2. K2O and Na2O increase 3. Al2O3 does not exhibit a strong variation. The graphs at the bottom of the handout visually present the data for the same suite of rocks and again exhibit the same relationships (Harker diagrams). Any element or oxide which exhibits a wide variation in abundance may be chosen as the abscissa (X axis) resulting in a similar set of diagrams, however individual analysis would not appear in the same sequence on each diagram. At the bottom of the handout the sequence for listing the samples by 1) decreasing CaO and 2) increasing Na2O is shown. SiO2 is generally chosen because it is the most abundant oxide in igneous rocks and exhibits a wide variation in composition. This type of graphical presentation is useful for large quantities of analytical data and yields an approximation of inter-element variations for a group of samples. No genetic link can be inferred from Harker diagrams, i.e. that the lowest SiO2 content present on the diagram represents the original or first liquid, for the group of samples presented, from which all other liquids were derived. Fractionation Indices To obtain a genetic link between analyses of a given suite of samples fractionation indices were developed. These indices attempt to the results of chemical analyses from an individual igneous suite into their correct evolutionary order. These indices are not realistic but several come close to such an order. 1. MgO Index This is used for basaltic rocks. Positive correlations are produced for Na2O, K2O, and P2O5 indicating enrichment in these oxides with successive liquids. Negative correlations result for CaO. 2. Mg-Fe Ratios Again used for basaltic rocks. These involve a ratio of Mg to Fe: MgO/MgO+FeO (ferrous) MgO/MgO+FeO+Fe2O3 (ferric) Mg/Mg+Fe (uses atomic proportions of the cations). 3. Normative Ab/Ab+An Based on the values of Na2O and CaO. Only good for rocks which crystallize plagioclase, not effected by mafic mineral formation. Generally applied to granites. The above three indices are only good for specific lithologies, and thus have a restricted application. Two fractionation indices, based on complex equations have been suggested for more comprehensive use. 1. Solidification Index (Kuno, 1959) SI = 100 MgO/(MgO+FeO+Fe2O3+Na2O+K2O) For basalts this is similar to Mg/Fe ratios due to the relatively poor alkali content. As fractionation progresses the residual liquids become enriched in alkaliis, thus Na2O and K2O contents offset the Mg-Fe index. For mafic rocks SI is high, for felsic rocks SI is low. 2. Differentiation Index (Thornton and tuttle, 1960) DI = normative Q+Or+Ab+Ne+Ks+Lc This is based on the normative analyis results. For mafic rocks DI will be low, because in normative calculation these minerals are minor. Felsic rocks DI will be high because these minerals are abundant in the norm. Triangular Variation Diagrams These diagrams visually present the variation in 3 chemical parameters. Two are commonly used: 1. AFM - Mainly for Mafic Rocks o A = Na2O + K2O o F = FeO (+Fe2O3) o M = MgO Plotted as either molecular or weight percent values. 2. Na2O - K2O - CaO - Mainly for Felsic Rocks Uses either the molecular or weight percent values for the three oxides listed. Data may be plotted as weight percent oxide or atomic percent of the cations. The disadvantage to this is that the absolute values of the analyses are not readliy determined. Fractionation Mechanisms Fractionation mechanisms relate the final composition observed in an igneous suite to the original, primary composition of the source material which yields the end product. For fractionation an Evolutionary link is implied, yet no specific process is suggested. DEFINED Formation of a variety of substances from an initially homogeneous, single parent material. Any mechanism which accomplishes this is a fractionation or differentiation mechanism. Every fractionation mechanism involves the migration or transport of atoms of a particular element relative to other elements, resulting in different bulk compositions formed from a single starting product. The composition of the source material producing the magma may effect the type and degree of fractionation the magma undegoes. The obvious manifestation of fractionation is the variation observed in the chemical composition for a single volcanic flow or pluton. We will look at three fractionation processes: 1. Liquid Immiscibility 2. Hybridization and Assimilation 3. Fractional Crystallization LIQUID IMMISCIBILITY Immiscibile - liquids can not be mixed, e.g. oil and vinegar Proposed to explain the juxtaposition of two distinctly different 'liquid' compositions with no intervening, intermediate composition. e.g. globular masses of granitic composition in a basaltic matrix. Varioles, spherulres ===> variolitic, spherilitic This process has been in and out of favour with petrologists for the past century. Liquid immiscibility has been proposed to have played a role in the formation, by fractionation, of: 1. Archaean volcanic rocks from the Abitibi Belt of NE Ontario and NW Quebec. 2. Lunar Mare Basalts. All Apollo missions returned samples exhibiting evidence for immiscibility. 3. Cretaceous deep sea basalts. In all of the above ==> basalt host with 'granitic' globules or varioles. Immiscibility has also been proposed and or observed for alkali intrusions, which may have two silicate liquids or a silicate and carbonate liquid, and mafic intrusions, which may have silicate and sulphide liquids HYBRIDIZATION AND ASSIMILATION A magma rising through the crust may assimilate country rock material as it passes from its source area to its site of crystallization. The assimilated material will change (contaminate) the chemical signature of the magma, resulting in a 'new' liquid, which when solidified will be distinctly different from the original, uncontaminated magma. Xenoliths and inclusions within a plutonic or volcanic rock provide evidence for the assimilation process. Assimilation is a thermodynamic process involving the following principles; Heat of solution = heat of melting + heat of mixing where: 1. Heat of solution is the ability of a magma to dissolve an inclusion. 2. Heat of melting is the heat capacity of of the solid phases plus the heat of crystallization of the minerals involved. 3. Heat of mixing is the heat required to mix the phases. Examples of Assimilation 1. For a basaltic liquid assimilating a granitic solid consisting of quartz, feldspar (plagioclase and alkali feldspar and biotite. Q, F and B will be melted, the heat for melting comes from the heat generated by the crystallization of olivine and pyroxene from the liquid, not from the temperature of the basaltic liquid. The granite inclusions will not be completely melted ====> partial melting. The end result is a basaltic andesite with inclusions of chewed up, partially digested granitic material. 2. For the reverse process where granitic liquid incorporates basalt, the anhydrous minerals in the basalt (olivine, pyroxene and plagioclase) become altered to micas, amphiboles and epidote by the addition of H2O. The heats of crystallization of quartz, feldspar and biotite from a granitic liquid are not large enough to melt the basalt inclusions, resulting in very minor changes in the original liquid composition. The result is a granite with amphibolite inclusions. Any changes caused by assimilation and hybridization are dependant on the nature of the inclusions and the nature of the magmatic liquid into which the inclusions are emplaced. FRACTIONAL CRYSTALLIZATION Charles Darwin in 1844 first suggested the possibility that fractional crystallization playsa role in the formation of igneous rocks. This is the process by which solids, generally crytals, which form from a liquid are prevented from reacting with the liquid. Evidence for Fractional Crystallization 1. Observed changes in bulk composition of liquid, e.g. a single volcanic flow or within a single volcano. 2. Zoning in minerals - most silicate minerals crystallized in igneous systems exhibit evidence of zonation, which reflect changes in composition. 3. Reaction Rims - produced by chemical reaction between crystals and liquid or sudden changes in T and P. e.g. pyroxene rims on olivine result when the liquid containing the olivine becomes saturated with respect to silica as a result of the growth of olivine. Mg2SiO4 + SiO2 ===> 2MgSiO3 In fractional crystallization the solids are removed or isolated from the liquid, resulting in the remaining or residual liquid having a new composition. e.g. Basalt liquid crystallizes olivine (Mg2SiO4) which is undersaturated with respect to SiO2, causing the initial liquid in to become depleted in Mg and enriched in Si, resulting in less Mg and more Si in the liquid, after forming the olivine. If the olivine is now removed from the system, the residual liquid is now depleted in Mg and enriched in Si compared with the parent liquid. Many mechanisms of fractional crystallization have been proposed. Gravitational Effects This is the most often suggested mechanism which is interpreted to indicate that fractional crystalization has occurred. This is dependant on the density of the solid phase(s) and the density of the liquid phase from which the solids are crystillzing. May have crystal settling or floatation as a means of fractionation. Crystal Settling Most often cited gravitational effect in the recorded in the published literature. Early formed minerals olivine (3.3-3.4 g/cc) and pyroxene (3.2-3.5 g/cc) are generally denser than the liquid (3.0 g/cc) from which they crystallize. Due to the density contrast between liquid and solid, the solids settle out of the liquid. Evidence for settling has been observed in a variety of environments from a single lava flow, ~ 1 m thick, to plutons, 1,000's of metres thick. e.g. Palisades Sill (Triassic Age), outcrops along the west bank of the Hudson River, in New Jersey and has thickness that varies along its length from 230-365 m. At or near the base of the sill is an approximately 2.5 m thick olivine-rich layer (with 20% olivine). This olivine has been interpreted to have been concentrated by crystal settling from the overlying liquid, early in the crystallization process. Calculations show that the olivine-rich layer accumulated in over approximately 7,000 hours (290 days). Crystal Floatation This aspect of fractional crystallization has been demonstrated experimentally by Walker and Hayes (1977) and Campbell et al. (1979), for basaltic liquids (3.0 g/cc). Plagioclase (2.6-2.7 g/cc) has been shown to accumulate at the top of the liquid by floatation. Observed in volcanic flows, with plagioclase laths concentrated at or near the top of the flow. One other Fractional crystallization mechanism. Convection Effects This is interpreted to be due to P and T gradients within the magma chamber as convection currents carry solid material through the chamber where they experience various temperature and pressure variations. Liquid circulates within the chamber Crystallization occurs in the cool portion of the cell Absorption occurs in the hot portion of the cell Result is zoned crystals if the absorption is incomplete Convection is only effective when the volume of liquid is much greater than the volume of solid. INTRODUCTION Phase diagrams provide a graphical means of presenting the results of experimental studies of complex natural processes, such that at a given temperature and pressure for a specific system at equilibrium the phase or phases present can be determined. SYSTEM - Any portion of the universe which is of interest and can be studied experimentally. PHASE- any particular portion of a system, which is physically homogeneous, has a specific composition, and can be mechanically removed or separated from any other phase in the system. e.g. A system containing a mixture of ol and pl in equilibrium contains two phases - ol and pl. In petrology we generally deal with primary phases - any crystalline phase which can coexist with liquid, i.e. it formed/crystallized directly from the liquid. EQUILIBRIUM - The condition of minimum energy for the system such that the state of a reaction will not change with time provided that pressure and temperature are kept constant. In experimental petrology there are three practical criteria used to test for equilibrium. 1. Time - with time the system does not change its physical or chemical makeup. 2. Approach equilibrium from two directions, e.g. the melting point of Albite. o begin with a liquid of Ab composition (Na2O-Al2O3-6SiO2) and cool until Ab crystallizes - T=1100°C o begin with the same misture of solid Albite and heat it up until liquid forms - T=1120°C Melting point of albite = 1110°C + 10°C. 3. Attainment of equilibrium by using different reactants and procedures. To determine the melting temperature of Albite o grind up a sample of pure albite o combine powdered oxides to give pure Ab composition Use both to determine Ab melting point. One final term to be defined prior to examining phase diagrams. COMPONENT - the smallest number of independant variable chemical constituents necessary to define any phase in the system. components may be oxides, elements or minerals, dependant on the system being examined. For example, experiments carried out in the H2O system, show that the phases which appear over a wide temperature and pressure range are ice, liquid water and water vapour. The composition of each phase is H2O and only one chemical parameter or component is required to describe the composition of each phase. UNARY SYSTEMS Systems which can be defined by a single component are called Unary Systems. H2O System In this system pressures from 0 to 15 kbars seven phases, each with the same compostion - H2O have been recognized: Ice I Ice II Ice III Ice IV Ice V Water Steam SiO2 System In the one component SiO2 system in the temperature range from 0 to 2,000°C and a pressure range from 0 to 30 kbars six phases of SiO2 are recognized. At pressures > 30 kbar a seventh phase, stishovite, exists. The six phases of SiO2 are: coesite alpha quartz (Trigonal) beta quartz (hexagonal) tridymite cristobalite anhydrous melt THE PHASE RULE For a system at equilibrium the phase rule relates: P = number of phases that can coexist, to C = number of components making up the phases, and F = degrees of freedom. Where these three variables are related in the equation P+F=C+2 The degrees of freedom represent the environmental conditions which can be independantly varied without changing the number of phases in the system. Conditions include: Temperature, Pressure, Chemical Composition, pH, Eh, Oxygen Fugacity. Applying the phase rule to the simplified one component H2O system. Phase rule becomes P + F = 3, because we are dealing with a one component system (C = 1). 1. At A o o o P = 1 - water F = 2 - two degrees of freedom, So to maintain equlibrium, i.e. have one phase (water) stable temperature and pressure may vary independantly. 2. At B Point B lies on the boundary curve separating Ice from steam field, o P = 2 - Ice and Steam, o F = 1 - one degree of freedom, o To maintain equilibrium, i.e., to keep the 2 phases stable, must choose an arbitrary value for pressure or temperature, which will automatically fix either temperature or pressure. 3. At T o P = 3 - Water, Ice, Steam o F = 0 - No degrees of freedom, o All three phases coexist at equilibrium. Can not change pressure or temperature without causing the system to move away from Point T, which will cause one or more of the stable phases to disappear. Therefore the maximum number of phase which can stably coexist in a one component system is three, and they do so only if there are no degrees of freedom. INTRODUCTION TWO COMPONENT OR BINARY SYSTEMS On a two component or binary diagram three, variables can be represented. These are generally pressure (P), temperature (T) and compositional changes (X) on a two dimensional diagram. To graphically represent a Binary diagram one variable must be kept constant, which is usually P, and the phase diagram is plotted as a T-X diagram for a specific Pressure. At constant P there are 8 possible types of Binary Diagrams which are of interest to petrologist. Binary Diagrams Examples I Binary Diagrams Examples II We will look at crystallization and melting relationships in 3 of these types. 1. Simple Eutectic, no solid solution (#1 on Examples I) 2. Incongruently melting phase, No SS (#3 on examples I) 3. Complete to Partial Solid Solution (#5, #6 and #7 on Examples II). DEFINITIONS Fundamental Definitions The following are some fundamental definitions pertinent to the study of phase diagrams. 1. System: Any particular portion of the universe in which we are interested. 2. Closed System: A system isolated from the rest of the universe with respect to matter entering or leaving the system. Can however be heat (energy) transfer across boundaries of such a system. 3. Open System: A system not isolated from the rest of the universe in which matter can move in and/or out of the system. 4. Component: The smallest number of independent variable chemical constituents necessary to define any phase in the system. 5. Phase: Any particular part of a system physically homogeneous in itself, but which can be separated mechanically from any other phase of the system. 6. Homogeneous Reaction: Occurs within a single phase in a system. 7. Heterogeneous Reaction: Occurs within more than one phase in a system. 8. Equilibrium: The condition of minimum energy for the system such that the state of a reaction will not change with time provided P & T are kept constant. 9. Stability: The condition of minimum energy. 10. Instability: The condition where energy is not at a minimum. 11. Metastability: The condition where the energy of the system exceeds that of one or more other possible configurations of the system. 12. Degrees of Freedom (or Variance of a System): The number of independent variables which must be arbitrarily fixed in order to define a system completely. In petrology T, P and X (concentration) are most important. INTRODUCTION GETTING STARTED With reference to the hypothetical binary diagram the following terms are indicated: 1. Eutectic (Point E) - lowest temperature point on a T-X (Isobaric = constant P) diagram at which liquid coexists with solid. On this diagram the eutectic is the point of intersection between the liquidus and solidus. 2. Liquidus - surface on a phase diagram above which no solids exist. 3. Solidus - surface or line on a phase diagram below which no liquid exists. In this case the solidus is a straight line, representing a constant T = isotherm. In this system the two components which define this system are A and B. From these two components, three phases can coexist - Solid A, Solid B and Liquid. The two phases A and B, both of fixed composition, i.e. the phase compositions are not part of a solid solution series and can be determined using analytical techniques. Below the solidus only solids are present, in this case mixtures of A and B. PHASE RULE Phase Rule as applied to Binary Diagrams Binary diagrams are drawn for atmospheric pressures, thus P is no longer a variable and the Phase Rule becomes: P + F = C + 1 (Condensed Phase Rule) With reference to the hypothetical binary diagram: 1. At X Point X lies within the liquid field, o P = 1 - Liquid o C = 2 - two components A and B o F = 2 - two degrees of freedom, giving a divariant area. o To maintain equilibrium, i.e. to keep the single phase Liquid stable, T and X may be varied independently. 2. At Y Point Y lies on the boundary curve between the fields of Liquid and A + Liq. o P = 2 - Solid A and Liquid o C = 2 - two components A and B, o F = 1 - one degree of freedom yielding a univariant curve. o To maintain equilibrium, i.e. to stay on the boundary curve, Fixing T automatically fixes X. 3. At E Point E is the eutectic, the point where the liquidus and solidus intersect. o P = 3 - Solid A, Solid B and Liquid, o C = 2 - two components A and B, o F = 0 - no degrees of freedom, an invariant point. o No degrees of freedom, all 3 phases coexist in equilibrium, changing either temperature or compostion from the point represented by E will cause one or more of the phases to disappear, thus changing the value for P in the phase rule equation. LEVER RULE To determine compositions of phases and the relative proportions of phases to each other in Binary diagrams the LEVER RULE is used. Compositions by the Lever Rule Once again back to our hypothetical binary system 1. Point "I" lies above the liquidus within the liquid field. What is the composition, in terms of the two end member components, A and B, of the liquid represented by this point? To determine the composition of "I" you must complete the following steps: 1. Draw a line through "I" perpendicular to the AB join, i.e., the base of the diagram. This line represents a line of constant composition and is referred to as an isopleth. 2. The liquid at "I" consists of a mixture of A and B, the proportions of which can be determined simply by measuring the length of three lines, AI', BI' and AB and then ratio these lengths. %A = I'B/AB *100 %B = I'A/AB *100 This gives us the bulk composition of the liquid at this point. If the composition point for the moves then we get a new bulk composition for that point represented by the new liquid. With reference to the hypothetical binary system: 2. Point Z At the point represented by Z there are several questions to be considered: 1. What is the bulk composition of Point Z? Point Z has the same bulk composition as Point I, used above, as it lies on the same isopleth, but at a lower temperature. 2. What phases are present? Point Z lies in the field where two phases, B + L, are in equilibrium, therfore the two phases present have to be soild B and Liquid. 3. What are the proportions of the phases present? To determine the proportions of B + L at Z, carry out the following steps: 1. Draw a line through Z, parallel to the base of the diagram (This line is at a constant temperature and is an isotherm.) This line should extend only to the boundaries of the B + L field - Points X and Y. 2. Measure the three line segments - ZX, Zy and XY and ratio these lengths using the lever rule. % B = ZX/XY * 100 = 38% B %L = ZY/XY * 100 = 62% L 4. What are the composition of the phases present? At Point Z if we were to examine the system we would see crystals of B in a glassy matrix (the Liquid) in equilibrium. The points X and Y assist us in determining the compostions of the two phases. As this is the simplest binary system possible, one which does not exhibit solid solution, all of the solid phases are of fixed composition. Point Y lies on the right hand side of the binary system where we have 100% B and 0% A, therfore the solid represented by Y must have a composition of Pure B. The composition of the liquid in equilibrium with the Pure B is represented by X, on the liquidus surface. To determine the composition of the Liquid at X, draw an isopleth down to the base of the diagram. Now measure three line segments AX', BX' and AB, ratio these using the Lever Rule to get the composition of the liquid X, in terms of A and B, the two components which define the system. %A = BX'/AB *100 = 40% A %B = AX'/AB*100 = 60% B Liquid compositions are always expressed in terms of the two end member components which define the system. Likewise the composition of any solid phase (100% B, 0% A) and the Bulk composition (40% A, 60% B) of any liquid are also expressed in terms of the end member components. CRYSTALLIZATION IN BINARY SYSTEMS We will examine four separate binary systems which are pertinent to igneous petrology. With each of the systems examined we will consider: 1. EQUILIBRIUM CRYSTALLIZATION - where the liquid and crystals remain in contact with each other, and there is no change in the bulk chemical composition as a result of crystallization. 2. FRACTIONAL CRYSTALLIZATION - where the liquid and crystals do not remain in contact, the crystals are prevented from reacting with the liquid. The final liquid composition is not the same as the initial bulk chemical composition. EQUILIBRIUM CRYSTAZLLIZATION The Diopside - Anorthite System represents the simplest type of Binary diagram, and is one that contains a simple eutectic and no solid solution. This system is pertinent to the study of gabbroic rocks, which are composed essentially of clinopyroxene and plagioclase. The two components which define the system are: Diopside - CaMgSi2O6 Anorthite - CaAl2Si2O8 On the overhead note the following features: 1. melting temperatures of the pure end member components; 2. dilution effect of lowering the melting temperature of one end member by addition of the other; 3. eutectic - lowest temperature point on the liquidus surface, where three phases coexist. Equilibrium Crystallization Composition X The bulk composition of X is 75% An and 25% Di. For equilibrium crystallization the final solid must have the same composition as the initial liquid, therefore the final solid will be a mixture of 75% An and 25% Di. 1. At 1600° X1 is 100% liquid. As the temperature drops from X1 to X2 the composition reaches the liquidus surface at a temperature of ~ 1500°C. 2. At X2 On the liquidus surface the liquid now begins to crystallize Pure An (of fixed composition) which is in equilibrium with liquid. At the temperature of the liquidus, for this composition, or system consists of ~ 1% An and 99% liquid. 3. At X3 Drop the temperature to 1400°C Point X3. At this point the system consists of An in equilibrium with Liquid - X3 lies in the field of An + L. o What are the proportions of An and L and what are the compositions of the two phases at X3? Proportions (by the lever rule) %An = X3L3/AnL3*100 = 47% %L = X3An/AnL3*100 = 53% Compositions Pure An - CaAl2Si2O8(100% An 0% Di) Liquid, represented by L3 situated on the liquidus surface % An = L3'Di/AnDi * 100 = 53% An % Di = L3'An/AnDi * 100 = 47% Di. 4. At X4 The system has reached the temperature of the eutectic 1270°C The liquid composition has moved down the liquidus surface from L3 to the eutectic. The composition of the eutectic liquid (LE) is 42% An and 58% Di. Notice that the liquid composition has changed from having 75% An to only having 42% An. This results from the fact that as the temperature dropped from 1500° to 1270°C An continuously crystallized from the liquid, thus depleting the liquid in the An component. Overall the bulk composition has remained the same, only the proportions and compositions of the phases involved have changed. At X4 the proportion of An:L is 56:44, applying the Lever rule to the line from An to E. The liquid remains at TE (1270°C) and now begins to crystallize Di along with An, in the proportions corresponding to the composition of the eutectic (42% An and 58% Di). Crstallization of An and Di continues until all of the liquid is used up. With the crystallization of the last droplet of liquid into grains of An and Di, the temperature of the system can now fall below TE. 5. At X5, T = 1250°C We have two solids An and Di in equilibrium with each other. The relative proportions of the two solids is the same as the bulk compositions X which we began with. For equilibrium crystallization the bulk composition we end up with is the same as the bulk composition we began with. The crystallization of a solid phase depletes the liquid in the components of that phase, thus changing the composition of the liquid in equilibrium with the solid. FRACTIONAL CRYSTALLIZATION Two types of fractional crystallization to consider: 1. Batch Fractionation - where crystals are removed from the liquid in a batch, i.e., more than one crystal involved. 2. Pure Fractionation - where each individual crystal, once it is formed is removed from the liquid, such that the liquid composition is constantly changing. Batch Fractionation Start with Composition X - 75% An - 25% Di. 1. From X1 to X2, temperature drops. 2. At X2, T = 1500°C, An begins to crystallize and is in equilibrium with liquid. 3. From X2 to X3, temperature continues to fall, An + L, the liquid composition moves down the liquidus, towards the eutectic, to maintain equlibrium with the solid An crystallized in the interval X2 to X3. The proportions of An:L are changing, An increasing and L decreasing, as a result of the An crystallizing from the liquid. 4. At X3 (T = 1400°C), we have An in equilibrium with liquid, proportions 47% An and 53% L. If we now fractionate, remove, all of the An crystals formed over the 100°C drop in T, from X2 to X3, we are left with liquid, L3. This liquid is referred to as a residual liquid as it the residue left over after An crystals were removed them from system. Once the An has been removed we are left with a liquid having a composition of 48% Di and 52% An. After removal of the An this 'new' liquid continues to cool. In the interval from L3 to X4, An crystallizes and the liquid composition once again moves down the liquidus surface towards the eutectic. 5. At X4, we have 19% An and 81% L in equilibrium with each other. If we now fractionate the An, we are left with a 'new' liquid with a composition represented by L4, (42% An and 58% Di). This new liquid once again cools, and as it cools it again crystallizes An from L4 to X5. The Liquid composition moves down the liquidus surface towards the eutectic. 6. At X5, at the temperature of the eutectic, Di begins to crystallize with An. The temperature of the system stays at the temperature of the eutectic until all the liquid is consumed. The final solid is a mixture of An and Di, in the proportions represented by the liquid composition L4 (the composition of the last residual liquid produced as a result of fractionation. Pure Fractionation Start with Composition X - 75% An - 25% Di For pure fractional crystallization, as each grain of An is formed it is removed from the system such that a residual liquid is produced, giving a new starting liquid which produces a new grain of An, which when removed yields a new residual liquid. The path followed during pure fractionation is a series of steps, with the drop representing the crystallization of An and the step representing the removal of the An. The result is that the liquid composition is constantly changing, in order to maintian equilibrium, moving down the liquidus surface towards the eutectic. Because the An is being constantly removed the overall system is becoming enriched in Di. The last liquid will have a composition of the eutectic and will yield a solid mixture of An and Di, in the proportions given by the eutectic. The bulk compostion of this solid will be the eutectic composition. MELTING We have just examined the effects of crystallizing liquid compositions in the AnDi system. What about the effects of melting solid material? Equlibrium Melting of Composition Y For equilibrium melting the liquid produced on melting stays in contact with the solid being melted, thus the final liquid will have the same bulk composition as the initial solid. For the melting path followed refer to the figure indicated in the above link. Fractional Melting of Composition Y For fractional melting (Pure melting is represented on the above link) the liquid produced on melting is removed from the system and is not abel to equilibrate with the residual solid. CONGRUENTLY MELTING INTERMEDIATE COMPOUND System 2 - Simple binary system with a congruently melting intermediate compound. Congruently Melting Compound - is one that when it is heated, will melt to a liquid of its own composition. In this system the congruently melting compound produces a thermal maximum. The result is that the system can be divided into two binary diagrams, each with a simple eutectic. Bulk compositions to the left of AB2 will produce final solid mixtures of A and AB2. Bulk compositions to the right of AB2 will produce final solid mixtures of B and AB2. INTRODUCTION System 3 - No SS, Incongruently Melting Compound Incongruently Melting Compound is a solid phase which when heated does not melt to a liquid of its own composition, it melts to a liquid and another solid, e.g. Enstatite ===> Forsterite + Liquid Both reactions take place at low pressure. Orthoclase ===> Leucite + Liquid We will look at the Forsterite - Silica system, in which Enstatite is the Incongruently Melting Compound. In examining the forsterite - silica system, at P = 1 atm., the following features are clearly evident: 1. En melts incongruently to a Liquid + Fo. 2. Liquid immiscibility at high temperature for silica-rich melts. In examining this same system at progressively increasing pressures the following changes in the appearence of the system are evident: 1. With increased pressure, from 1 atm to 7 kbar, En changes from incongruent to congruent melting behaviour. 2. The field of liquid immiscibility, the 2L field on the 1 atm diagram, dissappears at slightly elevated pressures and is no longer evident in the 3 kbar diagram. The Fo - Si system can be used to explain the incompatibility of olivine and quartz in igneous rocks and to simplify and model processes occurring in layered mafic intrusions, examples of which include the Skaergard Intrusion, Muskox Intrusion, Bushveld Intrusion. Simplified Fo-Si System The 1 atm system, represented in the above link, is not to scale in terms of temperature and is simplified to ignore the effects of liquid immiscibility at high silica contents. However on the diagram it can be seen that there exists: 1. a single binary eutectic (E) at a temperature = 1543°C 2. a binary peritectic (P) at a temperature = 1557°C. The temperature of the peritectic corresponds to temperature at which the following reaction occurs. En ===> liquid + Fo Peritectic - is defined as a low temperature inflection point on the liquidus surface at which a unique liquid of specified composition is in equilibrium with two or more crystalline phases. In this case the liquid has a composition of P and the two solids are En and Fo. EQUILIBRIUM MELTING Equilibrium Melting of Pure En Start with a solid of pure En, which is a mixture of the two components Fo and Si (60:40) which define the system. At a temperature below 1557°C only solid En exists. At a temperature above 1557°C the system consists of Fo + L, with a bulk composition equivalent to the starting composition of pure En, a 60:40 mixture of Fo and Si. This mixture of Fo + L results from the following reaction En ====> Fo +L which takes place at the temperature of 1557°C. At this temperature, represented by Point 3, 3 phases coexist, En, Fo and L. Applying the phase rule tells us that F=0, no degrees of freedom, making this point invariant. With the continued addition of heat to the system, in order to maintain equilibrium En decomposes into a mixture of Fo + L. The liquid is enriched in Si and is represented by point P, the peritectic, on the liquidus surface. As heat is continually added to the system, the system stays at the temperature of the peritectic until all of the En is melted, at which time we are left with a mixture of Fo + L. At temperatures above P as heat is added, the Fo begins to melt and is in equilibrium with liquid. As the Fo melts the liquid composition moves up the liquidus, becoming enriched in Fo and depleted in Si. When the temperature reaches the liquidus surface, the last grain of Fo is melted and we are left with a liquid, with a bulk composition equivalent to the initial solid - Pure En (Fo:Si = 60:40). The isotherm corresponding to the melting temperature of En and the Peritectic tells us that a reaction must occur in the system in order to maintain equilibrium. EQUILIBRIUM CRYSTALLIZATION Equilibrium Crystallization of Composition X Bulk composition of X is 80% Fo and 20% Si. Since this is equilibrium crystallization the final solid must also have the same bulk composition as the staring liquid and will be a mixture of Fo+En. This can be determined by drawing the isopleth from the initial liquid composition to the base of the diagram, where it lies in the stability field of Fo+En. 1. To begin Composition X lies above the liquidus and is 100% liquid. 2. Drop the temperature to the liquidus, Point X1, where Fo begins to crystallize and is in equilibrium with Liquid of composition X1. 3. As the temperature drops from X1 to X2, Fo continues to crystallize. Since Fo is being removed from the liquid, the liquid is becoming depleted in the Fo component and enriched in the Si component, thus it's composition moves down the liquidus surface, towards P, in the direction of increasing Si component. 4. At X3, the temperature of the peritectic, a reaction occurs where: 1. Fo, crystallized in the interval from X1 to X3, reacts with the liquid, of composition P, and is resorbed back into the liquid, and 2. En begins to crystallize. 5. Fo must compose a portion of the final solid, i.e. the isopleth extends into the Fo + En field, the resorbtion of Fo is incomplete - there is not enough liquid to react with the Fo. 6. As the Fo is resorbed, En crystallizes until all the liquid is used up. 7. The final liquid has a composition equivalent to the composition of P (52% Si, 48% Fo). 8. The final solid must be a mixture of Fo and En. The proportions of which can be determined by applying the lever rule to point X4. (50:50 mix of Fo:En) 9. The final solid has the same bulk composition as the initial starting liquid composition. Equilibrium Crystallization of Composition Y Bulk composition of Y is 50% Fo and 50% Si. Since this is equilibrium crystallization the final solid must also have the same bulk composition as the starting liquid and will be a mixture of En + Qtz. 1. To begin Composition Y lies above the liquidus and is 100% liquid. 2. Drop the temperature to the liquidus, Point Y1, where Fo begins to crystallize and is in equilibrium with Liquid of composition Y1. 3. As the temperature drops from Y1 to Y2, Fo continues to crystallize. Since Fo is being removed from the liquid, the liquid is becoming depleted in the Fo component and enriched in the Si component, thus it's composition moves down the liquidus surface, towards P, in the direction of increasing Si component. 4. At Y2 the system consists of a mixture of Fo+L. Applying the lever rule at Y2 shows that the proportion of Fo: L is 98:2. 5. As the temperature continues to drop from Y2 to the temperature of the peritectic Fo continues to crystallize. 6. At the temperature of P, the peritectic reaction takes place, where Fo + L ===> En. o Fo is resorbed into the liquid and En begins to crystallize. Both of theses solid phases are in equilibrium with liquid of composition P. There is an excess of L over Fo for this composition, i.e. all the Fo is resorbed and there is still liquid available. o With no Fo remaining the peritectic reaction no longer takes place and with a drop in temperature En crystallizes and we move into the field of En + L. 7. At Y3 the system is a mixture of En + L (64% En, 34% L). The liquid composition is moving down the liquidus surface towards E, the eutectic. 8. At the temperature of the eutectic, En continues to crystallize, Qtz begins to crystallize and these are in equilibrium with a liquid of eutectic composition. The temperature remains constant at 1543°C, until all the liquid is used up crystallizing into En and Qtz. These two phases crystallize out in the proportions given by the eutectic 45% En and 55% Qtz. 9. When the last drop of liquid crystallizes in to the last grains of En and Qtz we are left with a solid mixture of En+Qtz, in the proportions En:Qtz = 84:16, given by point Y4, with a bulk composition of 50% Fo and 50% Si, the original starting bulk composition. Equilibrium Crystallization of Composition Z Composition Z has a bulk composition of 40% Si and 60% Fo, equivalent to pure En, so our final solid must be 100% En, with the same bulk composition as the starting liquid. For this starting composition at the temperature of the peritectic the reaction En ===> Fo + L moves to the left crystallizing En. When the last grain of Fo is resorbed into the liquid the last droplet of liquid forms a grain of En, leaving a final solid of 100% En. The En isopleth essentially divides this system into two parts: 1. On the Fo side of En, the last liquid will have a composition represented by P, and will form a final solid mixture of En + Fo. 2. On the Qtz side of En, the last liquid will have a composition represented by E, and will form a final solid mixture of En + Qtz. In this type of system with a peritectic, there is also an incongruently melting compound, in this case En, which implies that a reaction relationship occurs between this compound the liquid and another solid phase. In this specific system Fo and Qtz will not occur together, they react to form En. This can be seen in the diagram where there is nor primary phase field where Fo and Qtz coexist. FRACTIONAL CRYSTALLIZATION Pure Fractional Crystallization For pure fractional crystallization, all initial liquids, regardless of their starting compositions, will yield a solid mixture of En + Qtz, in the proportions represented by the eutectic composition. The final liquid present following a path of pure fractional crystallization will have a composition of the eutectic Batch Fractional Crystallization For Batch fractional crystallization the composition of the last liquid and the solid phases present when crystallization has ceased will be dependant on where the last batch of crystals were removed along the path followed. In the example shown in the above link, fractionation occurs at X2 and X5, leaving a final liquid composition of X6 and a final solid mixture of En + Qtz. The fractionation at X2 removes forsterite and produces a dunite, fractionation at X5 removes En and results in a pyroxenite. The final solid mixture of En + Qtz produces a norite. COMPLETE SOLID SOLUTION Solid solution is defined as a mineral which exhibits a variable composition represented by the substitution of one chemical species for another, e.g.: 1. Mg for Fe in olivine 2. NaSi for CaAl in plagioclase. These two systems are the most applicable to geological examination of solid solution crystallization and melting. The Ab-An system The appearance of this system at first glance is totally different from any other binary system we have examined. The reason for this is due to the solid solution between the two end member components - albite and anorthite. The system contains a Liquidus and a Solidus. Above the liquidus only liquid exists, as in the other systems. Below the solidus only solids exist. In this phase field instead of having two distinct solids of fixed composition there exists a single solid of variable composition - plagioclase. Phase Rule Applying the phase rule to points 1, 2 and 3 shown on the above diagram, shows that: 1. Point 1 Lies above the liquidus within the liquid field. o P = 1 - Liquid o C = 2 - two components Ab and An o F = 2 - two degrees of freedom o To maintain equilibrium we can change temperature, increase or decrease, and change composition, more or less An, and still have only one phase (liquid) present. 2. Point 2 Lies in the field between the liquidus and solidus. o P = 2 - Plagioclase solid solution and Liquid o C = 2 - two components Ab and An o F = 1 - one degree of freedom o To maintain equilibrium we can change only temperature or composition, but not both, to stay within this field. If we fix the solid as plagioclase of a specific composition then the liquid which is in equilibrium with this plagioclase becomes fixed. 3. Point 3 Lies below the solidus in the field of PlagSS. o P = 1 - plagioclase solid solution o C = 2 - two components Ab and An o F = 2 - two degrees of freedom o As with Point 1, we can change temperature and composition and still have only one phase (plagioclase) present. EQUILIBRIUM CRYSTALLIZATION Equilibrium Crystallization of Composition X Point X has a bulk composition of 65% An - 35% Ab, which for equilibrium crystallization remains unchanged, so our final solid must be mixture of 65% An and 35% Ab. This can be determined by drawing the isopleth from our starting composition to the base of the diagram. 1. From X1 to X2 the liquid simply cools down to the liquidus surface. 2. At X2, on the liquidus surface. o Plagioclase begins to crystallize. o This plagioclase has a composition represented by Pl2 and is in equilibrium with L2. o Note that the an An-rich plagioclase is in equilibrium with a more Ab-rich liquid. 3. From X2 to X3 o The liquid composition moves down the liquidus from L2 to L3, becoming richer in the Ab component. o At the same time the plagioclase composition moves down the solidus from Pl2 to Pl3, becoming more Ab rich. o Again note that the liquid composition is still more Ab-rich than the solid. o In this type of system the horizontal lines from the liquidus to the solidus are isotherms, which join a liquid and solid phase which are in equilibrium with each other. o In order to maintain equilibrium between the solid and liquid as the temperature drops the composition of the solid and liquid phases is constantly changing. 4. At X4, on the solidus. o The last droplet of liquid, with a composition represented by L4, is in equilibrium with plagioclase Pl4, which has a composition of 65% An 35% Ab - the bulk composition of the initial liquid. Note in this system the composition of the plagioclase is always more enriched in the An component than the composition of the liquid which is in equilibrium with the solid. FRACTIONAL CRYSTALLIZATION Fractional Crystallization of X 1. Pure fractional crystallization of composition X As each grain of plagioclase is removed the residual liquid acts as a new initial liquid. The result is that for pure fractional crystallization the final solid will be a grain of plagioclase with a composition of 100% Ab - 0% An. 2. Batch fractional crystallization As each batch of plagioclase grains is removed a new liquid is left over and it begins to crystallize a new plagioclase of a composition represented by the points, Pl3, Pl4, and Pl5, where the solidus and the isotherm intersect. The final solid will be a mixture of An and Ab determined by the composition of the residual liquid after the last batch of crystals has been removed. The Ab- Or System This system is important in the study of granitic rocks. With the addition of Qtz to the Ab-Or system, it is a first approximation of a true granite. The appearance or topology of the Ab-Or system changes with pressure. At pressures < 4 kbars the system displays complete solid solution between Ab and Or. At pressures > 4 kbars the system displays partial solid solution. Another important aspect of the system is the presence of the solvus, below which the feldspar solid solution undergoes unmixing to produce two separate solid feldspars, one rich in the Ab component and one rich in the Or component. Overall the effects of increased pressure on this system are: 1. Disappearance of leucite from the binary diagram. Leucite is not stable at P > 2 kbars. Leucite is not found in plutonic rocks which have crystallized at depths corresponding to pressures in excess of 2 kbars. Leucite is most commonly found in volcanic rocks, which are undersaturated with respect to silica. 2. Increasing P also lowers the melting point of both end member components, Ab and Or. 3. Nature of the phase diagram changes - the minimum disappears, it is replaced by a eutectic, and the solid phases exhibit partial solid solution. For the 5 kbar diagram of this system, the results of experimental work are shown. This system has been determined using 20 mixtures (isopleths) of Ab and Or which were equilibrated at a variety of temperatures, then quenched followed by examination of the phases present. The experimental results are recorded on the diagram using the following symbols: 1. red circles for liquid, 2. green circles for liquid and solid, and 3. blue circles for solid. The isopleths are represented by the vertical columns of symbols. The gas phase is water vapour, which is present in excess, as water is used as the pressure medium. Look at the 2 kbar diagram in detail. Crystallization and melting paths in this system are comparable to those examined in the Ab-An system. As liquids cool between the liquidus and solidus the solid phase is constantly changing compostion, along the solidus, to maintian equilibrium with the liquid, which also is changing compostion, along the liquidus. The aspect of this diagram which is different from previous diagrams examined is the presence of the solvus, below which the feldspar solid solution unmixes to two separate feldspars, one qich in Ab and one rich in Or. This exsolution results in the formation of a variety of textures which can be recognized by examining the resulting rock under the microscope. 1. If the feldspar grain consists of an Or-rich host and exsolution lamellae of Ab, then the grain is a perthite and exhibits a perthitic texture. 2. If the feldspar grain consists of an Ab-rich host and lamellae of Or, the grain is an antiperthite, resulting in an antiperthitic texture. INTRODUCTION Ternary phase diagrams are 3 component systems. To construct a ternary diagram it is necessary to know the three binary systems for the three components. Ternary diagrams have a vertical temperature axis. The actual ternary diagram may be represented as a three dimensional form or more commmonly as a two dimensional projection of the liquidus surface onto the base of the triangle created when the three binary diagrams are joined together. This irregular triangle is often transformed into an equililateral triangle to facilitate presentation and interpretation. For experimentally studied ternary systems the liquidus surface may be contoured with the temperature interval representing the contour interval. The fields indicated on the ternary diagram represent the primary phase fields, of solid phases in equilibrium with liquid, present on the liquidus surface. The lines or curves which separate primary phase fields, the red, blue and magneta lines on the above image, are referred to as Cotectic Lines or Boundary Curves, along which 3 phases are in equilibrium - two solids and a liquid. Straight lines which join the composition points of two phases whose primary phase fields share a common boundary curve are called Alkemade Lines. Thus the edges of the triangle are Alkemade Lines as they join the individual phases AB, AC and BC, which share a boundary curve. Alkemade lines are a specific type of tie line PHASE RULE Applying the Phase Rule to Ternary Diagrams To refresh your memory the phase relates the number of phases present in the system, the the number of components which define the system and the degrees of freedom displayed the by the system at equilibrium. Phase Rule P + F = C + 1 With referernce to the hypothetical ABC system: 1. At Point 1 Point 1 lies in the C + L field o P = 2 - Solid C and Liquid o C = 3 - three components A, B and C o F = 2 - two degrees of freedom, giving a divariant surface o To maintain equilbrium, i.e. to keep Solid C and Liquid as the stable phases, temperature and composition may both vary independently of each other . 2. At Point 2 Point 2 lies on the Boundary Curve which separates the field of Solid A + LIquid from the field of Solid C + Liquid. o P = 3 - Solid A, Solid C and Liquid o C = 3 - three components, A, B and C o F = 1 - One degree of freedom, giving a univariant line. o To maintain equilibrium between the three phases i.e. to stay on the boundary curve, only temperature or pressure may be changed independently, fixing one automatically fixes the other. 3. At Point E Point E represents the ternary eutectic, where the three fields A + L, B + L and C + L meet. o P = 4 - Solid A, Solid B, Solid C and Liquid o C = 3 - three components, A, B and C o F = 0 - No degrees of freedom, yielding an invariant point. o To maintain equilibrium, i.e. to have all four phases stable and in euilibrium the position of the eutectic is fixed in terms of temperature and composition, changing either will result in a shift of the system away from the eutectic. DETERMINING COMPOSITION ON TERNARY DIAGRAMS Compositions in Ternary Diagrams All compositions, e.g., bulk compositions, liquid compositions, compositions of solid phases, on ternary diagrams are expressed in terms of the three end member components which define the system. These three components are located a the apices of the triangle. Each apex on the triangle representing the ternary system represents 100% of the component at that apex. The side of the triangle, directly opposite the apex, represents 0% of the apex component. Compositions of points which lie along the outside edge of the triangle are simply a mixture of the two components at each end of the tie line, with 0% of the third component. The composition of points which lie inside the area of the triangle can be determined by using either of the following methods. 1. Method 1 - Triangular Grid In this method a grid is constructed on the diagram. This grid is most commonly set up representing a 10% incremental increase in the components. To determine the composition of a point within the triangular area of the diagram a series of three lines are drawn through the point of intersest, with each line parallel to a side of the triangle. With these lines in place the percentage of each component in the compostion of the point can readliy be determined. 2. Method 2 - Two Line Method In this method only two lines are drawn, through the point of interest, parallel to any two sides of the triangle. The intersection of these two lines with the third side divides this side into three line segments. The lengths of the individual line segments are proportional to the relative amounts of the three components. The two methods outlined above assumed an equilateral triangle, however both methods can be applied when dealing with a non-equilateral or scalene triangle in nature. Determining Compositions To determine the composition of a point lying inside the triangular diagram, a series of three lines through the point of interest, with each line parallel to a side of the triangle are drawn, as shown on the above image. With these lines in place the composition of each point in terms of the end member components can be read directly from the diagram. On the overhead: 1. 2. 3. 4. Point E has a composition of 36% A, 43% B and 21% C. Point 1 has a composition of 19% A, 30% B and , 51% C. Point 2 has a composition of 45% A, 23% B and 32%C. Point e3 has a composition of 60% A, 0% B and 40% C. HYPOTHETICAL TERNARY SYSTEMS Hypothetical Ternary Systems - Without Solid Solution (a) congruently melting compound BC, 2 eutectics; (b) congruently melting compound, BC, 1 eutectic, 1 peritectic; (c) incongruently melting compound BC, 1 eutectic, 1 peritectic; (d) congruently melting compound ABC, 3 eutectics; (e) incongruently melting compound ABC, 1 eutectic, 1 distributary peritectic, 1 tributary peritectic. Hypothetical Ternary Systems - With Partial to Complete Solid Solution (a) limited solid solution of A with B or C (b) complete solid solution between B and C (c) congruently melting compound BC, in complete solid solution with A (d) incongruently melting compound BC in complete solid solution with A. Systems displaying complete solid solution will not contain a ternary eutectic. (e) complete solid solution between B and C, but with a ternary minimum (M) between the fields of A + L and BCSS + L. The BC binary system displays a binary minimum (m), as in the Ab-Or system. Instead of a eutectic in this system a thermal valley exists between M and m and represents the lowest temperature at which a liquid exists. Ternary Phase Diagrams - General Rules 1. A congruently melting compound will lie within the primary phase field for that compound. 2. An incongruently melting compound will not lie in the field of that compound. 3. Ternary diagrams which do not exhibit solid solution effects will contain at least one ternary eutectic, i.e. the lowest temperature at which liquid can exist. 4. In systems with no solid solution conjugation lines or Alkemade lines within such systems are thermal barriers. 5. Ternary systems which contain an incongruently melting compound will contain at least one ternary peritectic. 6. Ternary systems which exhibit complete solid solution effects will not contain a ternary eutectic within the triangular area representing the system. 7. For any ternary diagram identify all Alkemade lines. For every boundary curve there exists an Alkemade line. 8. Consider the relation between a boundary curve and its pertinent Alkemade line; o i) The actual or projected intersections between boundary curve and its pertinent Alkemade line represents a temperature maximum of the boundary curve. o ii) If tangents drawn from the boundary curve intersect the pertinent Alkemade line, then that part of the boundary curve is a subtraction curve. o iii) If tangents drawn from the boundary curve intersect an extension of the pertinent Alkemade line, then that part of the boundary curve is a reaction curve. 9. After the thermal slopes, i.e. the direction of falling temperature, of the boundary curves are determined, the various ternary invariant points can be identified as either eutectic, tributary or distributary reaction points. DEFINITIONS Subtraction Curve - That part of the boundary curve along which crystalline material precipitates from the liquid. Reaction Curve - That part of the boundary curve along which crystalline materials both precipitate and dissolve. DIRECTIONS OF FALLING TEMPERATURE For ternary systems where actual isotherms are not known it is still possible to determine the dirctions that temperatures will fall by examining the relationship between tie lines and boundary curves. Determining the directions of falling temperature 1. Temperatures fall away from the three apices along the edges of the triangle. 2. The point of intersection of a tie line joining two compounds, that share a boundary curve, with the boundary curve or an extension of the curve represents a thermal maximum along the pertinent section of the boundary curve. These relationships can be illustrated by examing the hypothetical ABC system. 1. The primary fields A + L and B + L share a boundary curve, and the tie line or Alkemade line joining the two solid phase is the AB side of the triangle, therefore the temperature along the boundary curve separating the A + L field from the B + L field must move away from the point of intersection along the boundary curve. 2. The dashed line joining A and BC represents an Alkemade line, which crosses the boundary curve between the A + L and BC + L fields. Therefore the point of intersection is a temperature maximum, and temperatures fall away, in both direction, from the point of intersection. 3. Compound BC is congruently melting, its bulk composition point lies within the primary phase field of BC + L. This system exhibits two ternary eutectics, the arrows along the boundary curves converge at the eutectics. Ternary peritectics occur in systems containing both congruent and incongruently melting compounds. At ternary peritectics, the following relationships of boundary curves are possible: 1. tributary - where two boundary curves come into the triple point and one goes out, or 2. distributary - where one boundary curve comes into the triple point and two curves go out. ISOTHERMAL SECTIONS One method of examining the phase relationships within a ternary system is by the construction of isothermal sections through the diagram, parallel to the base. An isothermal section is a representation of the stable phases for different compostions which have been quenched to the same temperature. The isothermal sections to be examined are derived from the liquidus diagram, where we are looking at the phases present between the liquidus and the solidus. The best way to view Isothermal Sections is by examining a simple ternary system. 1. Simple Ternary System A simple ternary system with a single eutectic. The individual isotherms (in blue) are labelled T5 through T1, such that T5 > T4 > T3 > T2 > T1. The cotectic curves (in magenta) separate the three primary phase fields A + L, B + L and C + L from each other. 2. Isothermal Section at T > temperature of melting of Pure B (TB) In this section, made at a temperature above the highest temperature on the diagram, so we are above the liquidus, there exists a single "one-phase field" consisting entirely of melts throughout the system, regardless of the compostion. Another way of looking at this is that no matter what the composition of the system, it will be 100% melt. 3. T5 Isotherm For this section, which is at a temperature that intersects the liquidus but not a cotectic curve, the one-phase melt field has become smaller and we have the addition of two "two-phase fields": B + Liq and C + Liq. The boundaries of the melt field are the appropriate contour line representing the isotherm for this section. In each of the two-phase fields, radiating tie lines (in red) are shown connecting coexisting melt and crystal compositions. These tie lines link solid crystals, of fixed composition, (the point from which the tie lines radiate) with liquid of a variety of compositions, represented along the liquidus. 4. T4 Isotherm The one-phase melt field has decreased in size, as the temperature of the 5. 6. 7. 8. iosthermal section is lowered. At the same time the two-phase fields of B + Liq and C + Liq have increased and been joined by the two-phase field A + Liq. T3 Isotherm The one-phase field continues to shrink while the three two-phase fields increse in size. T2 Isotherm In this isothermal section the isotherm has intersected the cotectic curves (in magenta) as well as the liquidus surface. In addition to the one-phase melt field, which continues to decrease in size, and the three two-phase fields, three threephase fields are present. In each three-phase field a melt of fixed composition (on the cotectic) and crystals of two solids coexist, e.g. A + B + Liq, A + C + Liq and C + B + Liq. Note that only one melt composition can exist in a three-phase field, whereas any number of melts (on the contour line along the boundary of teh melt field) can exist in a two-phase field. T1 Isotherm The one-phase melt field has decreased, the two-phase fields have gotten smaller and the three-phase fields have increased. Isothermal section at T < temperature of the eutectic (TE) For this isothermal section, which is at a temperature below the temperature of the eutectic, i.e. below the solidus, then the entire diagram is a three-phase field, A + B + C, with no melt remaining and the system at every compostion will consist entirely of some combination of A, B and C. INTRODUCTION Ternary System #1 - An-Wo-Sp System System 1 - Anorthite - Wollastonite - Sphene System This is a simple ternary system, with no solid solution, exhibiting a single ternary eutectic. Along the edges of the triangle there exists a binary eutectic in each of the simple binary systems, e.g. Wo-An, Wo-Sp and An-Sp. The three components which define the system are: Anorthite - CaAl2Si2O8 Wollastonite - CaSiO3 Sphene - CaSiTiO5 On the image above note that the liquidus surface slopes down from each apex of the triangle towards the eutectic inside the diagram. Any liquids present in the system and which undergo crystallization will travel down the liquidus surface towards the eutectic. On ternary systems we will be tracing out the path the liquid follows, and as we will see it is possible to predict the path any liquid will follow during crystallization. Remember you are looking at a map of the liquidus surface so that the fields present are labelled as a solid + liquid, e.g. Wo + L. The cotectic boundaries, shown in purple, separate the individual fields from each other. The isotherms are labelled in red and marked as dashed blue lines. EQUILIBRIUM CRYSTALLIZATION Composition X Since we are examining equilibrium crystallization, the final solid must have the same bulk compostion as the initial liquid. The bulk composition of X, in tems of the three end member components which define the system, is 60% Wo, 20% An, 20% Sp. The compostion of X is determined by drawing a series of lines (the red lines) through X parallel to the sides of the triangle and the corresponding percentage of each component can be calculated. As these are the only phases present in this ternary system and all three coexist at the ternary eutectic, the liquid must move to the eutectic. The final liquid will be a mixture of An Wo and Sp, in the proportions of the eutectic. So we know what our initial liquid composition is and we know what our final liquid composition will be - What path will the liquid follow? The path followed by the liquid is shown as the blue line on the image. 1. To begin, X is at some temperature well above the liquidus and as such is 100% liquid. As the temperature drops to the liquidus the system still consists of 100% liquid 0% solid. 2. Upon cooling to the liquidus, the bulk composition does not change, and reaches the liquidus at point X, which lies in the field of Wo + L. At this point Wo begins to crystallize and it is in equilibrium with liquid. Due to the crystallization of Wo, the liquid composition must change, i.e., it is becoming depleted in the Wo component and enriched in the An and Sp components. The result is that the liquid composition moves directly away from the Wo composition point. 3. As the liquid moves away from X towards Z, it is changing in the following way: o It is becoming depleted in Wo. Wo is being removed from the liquid into solid Wo crystals, and o It is becoming enriched in An and Sp, it is moving towards the An-Sp side of the diagram. 4. At Point Z The two phases in equilibrium are Wo and L. Two questions to ask here are: 1. What are the proportions of Wo and L at Z? 2. What are the compostions of Wo and L at Z? Proportions Applying the lever rule at Z will provide the proportions of Wo and L. o o % Wo = XZ/WoZ *100 = 24% % L = WoX/WoZ * 100 = 76% Compositions The composition of the two phases Wo and L at Z, must be expressed in terms of the three end member components of the ternary diagram An, Wo and Sp. o o The solid Wo is pure Wo (100%Wo, 0% An, 0% Sp). The liquid composition can be determined by drawing a series of three lines through Z, with each line parallel to one side of the triangle. This gives a composition for the liquid of 47% Wo, 27% Sp and 25% An. 5. As crystallization continues from Z to P, Wo continues to crystallize and the liquid continues to move directly away from Wo. 6. At P, which lies on the cotectic between the field of Wo + L and Sp + L. Sp begins to crystallize along with the Wo, because the liquid has reached the boundary curve between the fields of Wo + L and Sp + L. The result is that the liquid composition now moves down the boundary curve towards E as both Wo and Sp are removed from the liquid. 7. At Q Here three phases are in equilibrium, Wo + Sp + L. The proportion of solid (Wo+Sp) to liquid (L) and the composition of the solid and liquid phases can be determined. In order to calculate these values we must first draw a line from Q, the point of interested, through X, our bulk composition, back to intersect the edge of the triangle. This line on the image extends from Q through X to T. Proportions of Solid to Liquid o % Solid (Wo+Sp) = QX/QT * 100 = 45% o % Liquid (L) = TX/QT *100 = 55% Proportion of Wo to Sp o o % Wo = TSp/WoSp * 100 = 89% % Sp = TWo/WoSp * 100 = 11%. Compositions of Phases o o o Solid Wo is pure Wo (100% Wo, 0% Sp, 0% An) Solid Sp is pure Sp (0%Wo, 100% Sp, 0% An) The liquid composition can be determined by drawing a series of lines through Q, with each line parallel to one side of the triangle. 8. Crystallization of Wo and Sp together continues from Q to E. 9. At E, the ternary eutectic Wo and Sp continue to crystallize and are joined by An, with all three crystallizing together, in the proportions given by the eutectic. The system stays at the temperature of the eutectic until the last droplet of liquid crystallizes into the last grains of Wo, Sp and An. The compostion of the last liquid last liquid to exist in the system is represented by E. The proportions of the solid phases in the final solid after the last liquid has crystallized is given by X (60% Wo, 20% Sp, 20% An) - OUR INITIAL BULK COMPOSITION! In order to maintain equilibrium as the solid phases are crystallized the liquid composition is constantly changing as represented by the path from X to P to E, the solid blue line on the diagram. FRACTIONAL CRYSTALLIZATION Composition X For fractional crystallization of composition X, the path followed by the liquid is the same as the path followed during equilibrium crystallization. The only difference is that the final solid mixture will consist of Wo, Sp and An in the proportions given by the bulk composition of the last residual liquid remaining after the removal of solid crystals prior to the liquid reaching the eutectic. INTRODUCTION The Forsterite - Anorthite - Silica System This system exhibits an incongruently melting compound (En) along the binary join Fo-Si. This system also exhibits a ternary eutectic and a ternary peritectic. Things to note about this system are: 1. Melting and crystallization relationships in this system are ternary except along the Fo-An join where spinel appears on the liquidus. 2. The En-An join or tie line (dashed green line) divides the triangular area of the Fo-An-Si diagram into two smaller triangular areas: o Fo-En-An triangle o En-Si-An triangle. These two triangles (shown on the inset of the diagram) tell us something about the final solid compositions that will be present as a result of crystallization of a liquid and whether that liquid will move to the peritectic or eutectic as a result of crystallization. At the peritectic and the eutectic the three phases in equilibrium with liquid are Fo + En + An and En + Si +An, respectively, - the same phases that make up the two small triangles. o For equilibrium crystallization any staring composition that begins in the Fo-En-An triangle will move to the peritectic, where crystallization will cease and the last liquid will have a composition of the peritectic. o Compositions that start in the En-Si-An triangle will move to the eutectic, where crystallization will cease and the last liquid will have a composition of the eutectic. 3. Also in the system the boundary curve separating the fields of Fo + L from En + L is a reaction curve, labelled with a double arrow, and involves a reaction relationship during crystallization: En ===> Fo + L This is the same reaction we studied when examining the binary Fo - Qtz system. EQUILIBRIUM CRYSTALLIZATION 1 Composition X Point X The bulk composition of X can be determined by drawing three lines ( the magenta lines) through X, parallel to each side of the large triangle Fo-An-Si. Using this method we get a compostions of 70% Fo, 18% Si and 12% An. For equilbrium crystallization the final product must have the same bulk composition as the initial starting composition. Point X also lies in the small triangle Fo-En-An, therefore the final solid must be a mixture of the three phase that define this small triangle. The proportions of these three phases, present at X, can be determined again by drawing three lines ( the dashed black lines) through X, but parallel to the sides of the Fo-An-En triangle. The final solid will be a mixture of 28% Fo, 60% En and 12% An. These three phase in the final solid coexist at P, the peritectic, so the last liquid will have a composition equivalent to that of the peritectic. The path followed by the liquid is shown in red, with the sequence of events as follows: 1. Cool the liquid down until it reaches the liquidus. 2. At the liquidus Fo begins to crystallize and the Fo is in equilibrium with liquid. The liquid composition moves directly away from Fo, as it is being depleted in Fo. 3. From X to Y, Fo continues to crystallize and the liquid moves along the path, represented by the red line, constantly changing composition. 4. At Y, which lies on the boundary curve between the fields of En + L and Fo +L. This cotectic curve is a reaction curve denoted by the double arrow. The reaction taking place is Fo====> En + L At Y Fo is resorbed, melted back into the liquid and En begins to crystallize with both phases in equilibrium with liquid, represented by Y. 5. From Y to Point Q Fo continues to be resorbed, En crystallizes and both of these are in equilibrium with the liquid, the composition of which is moving down the boundary curve from Y to Q. 6. At Q Point Q lies along the cotectic curve separating the En + L field from the Fo + L field. At Q we can determine: 1. The proportion of solid Fo + En to L. To do this we must draw a tie line from Q through X, back to the En-Fo join (Point A). % Solid (Fo+En) = XQ/AQ * 100 = 64% We can also determine the ratio or Fo:En at Q % Fo = AEn/FoEn * 100 = 52% % En = FoA/FoEn *100 = 48% % L = AX/AQ * 100 = 36% 2. The proportion of F being melted and En crystallizing. To do this we must draw a line which is tangential to the cotectic curve from Q back to intersect the the extension of the Fo-En tie line - Line segment QC. Ratio of Fo being resorbed: En crystallizing = EnC/FoC = 1:1.65. From this ratio it can be seen that much more En is forming than Fo being resorbed. 3. The composition of all phases present. Fo is Pure Fo (100% Fo, 0% Si, 0% An) En is pure En (68% Fo, 32% Si, 0% An) Liquid composition is represented by point Q - draw three lines through Q parallel to the outside edges of the large triangle and get a compostion in terms of the three end member components Fo, Si and An. I haven't done it, you know how to, give it a try! 7. From Q to the peritectic (P). Fo resorbtion continues as does En crystallization. 8. At the peritectic Fo continues to be resorbed, En continues to crystallize and An begins to crystallize. The liquid stays at the point represented by the peritectic until all the liquid has been used up, leaving a solid mixture of Fo + En + An, in the proportions 26:60:12 - the proportions we calculated above using the small Fo-En-An triangle. EQUILIBRIUM CRYSTALLIZATION 2 EQUILIBRIUM CRYSTALLIZATION OF Z Composition Z lies on the En-An join, therefore the final solid must be a mixture of En and An in the proportions En:An = 70:30 - NO Fo or Si in the final solid. For equilibrium crystallization of Z, the final liquid is situated at P and it behaves like composition X previously looked at. At P, Fo is being resorbed and En and An are crystallizing from a liquid with a composition of the peritectic. This resorbtion and crystallization contiunes until the last grain of Fo is resorbed into the last droplet of liquid which crystallizes into the last grains of En and An. EQUILIBRIUM CRYSTALLIZATION OF A Composition A lies in the En-Si-An triangle, so it's last liquid must lie at E, the eutectic, where En + Si +an are in equilibrium. As this composition is initially in the Fo + L field, once it reaches the liquidus, it will begin to crystallize Fo and move directly away from Fo towards the En-Fo boundary curve. At the boundary curve the Fo will be resorbed and the En will crystallize, with the liquid composition moving down the boundary curve towards P. The proportion of Fo to be resorbed is very small, so at some point along the boundary curve all the Fo will be removed. This point can be located by drawing a line through A from En to intersect the boundary curve ( Point R). At this point all the Fo is resorbed, but En continues to crystallize, so the liquid now moves off the boundary curve, directly away from En into the En + L field. Crystallization of En continues until the boundary curve between the fields of En + L and An + L is reached (Point S), where An begins to crystallize along with the En. The liquid moves down the boundary curve from S to E. At E En and An continue to crystallize and are joined by Si. The liquid composition stays at E until all the liquid is used up and the final solid that results is a mixture of En-An-Si. EQUILIBRIUM CRYSTALLIZATION OF W Composition W lies in the En + L field, so at the liquidus temperature En crystallizes and the liquid moves directly away from En to the boundary curve between En + L and Si +L (Point V), where Si joins En in crystallizing. The liquid moves down the boundary curve from V to E, where An joins En and Si in crystallizing. At E all three solids phases crystallize until all the liquid is used up. The location of Composition W in the small triangle En-An-Si tells us that the final solid must be a mixture of these three phases. It should be noted that on the phase diagram that nowhere do Fo and Si coexist together. FRACTIONAL CRYSTALLIZATION Batch Fractionation The paths followed for liquids which undergo batch fractional crystallization will be dependant on where the individual batches of crystals are removed from the system and the composition of the residual liquid after removal. For Composition X if fractionation occurs between X and Z, the removal of the Fo crystals will yield a dunite, leaving a residual liquid of composition Z. If this is the only place where fractionation occurs then the last liquid will end up at P, leaving a solid mixture of En and An. If crystallization continues from Z to Q, Fo and En will be removed producing a peridotite, which is either olivine-rich or enstatite-rich, depending on which phase is dominant. By fractionating at Q, the liquid now moves off the boundary curve directly away from En, towards A. From Q to A, En crystallizes and the liquid composition moves away from Q. At A, En and An crystallize together and the liquid moves down the boundary curve towards E, where Si joins En and An, giving a solid which has enstatite anorthite and quartz - a quartz norite. The bulk composition of the final solid is given by point Q, the position where the last batch of crystals were removed. Pure Fractionation The paths followed for liquids which undergo pure fractional crystallization will all end at the eutectic, regardless of the initial liquid composition. For Composition X the final liquid remaining after pure fractional crystallization will have the composition of the eutectic. The final solid will be a mixture of Si + En + An, in the proportions given by E in the En-Si-An triangle. COMPOSITIONS, PROPORTIONS AND TIE LINES System Displaying Solid Solution The system Di-Ab-An will be examined. In this system liquids undergoing crystallization will produce two-phase assemblages: 1. diopside of fixed composition, and; 2. plagioclase of variable composition. No three phase assemblage of solids + liquid is possible as in the previous ternary systems examined. This is because of the complete solid solution between Ab and An, which yields plagioclase, of variable composition, and the lack of a ternary eutectic. Compositions and Proportions Start with an initial liquid represented by Point A. Point A has a bulk composition of 50% Di, 23% Ab and 27% An, determined by drawing a series of lines (the magenta lines) through A parallel to the outer edges of the triangle. On cooling, Liquid A will result in a solid mixture of Di and Pl, the proportions of which can be determined by applying the lever rule to a line drawn from Di, through A to the Ab-An join (Point B): % Di = AB/DiB * 100 = % Pl = ADi/DiB *100 = The composition of the Di is fixed at the composition point of Di (100% Di, 0% Ab and 0% An). The composition of the Pl is determined along the Ab-An join at Point B, again by applying the lever rule: % An = AbB/AbAn *100 = % Ab = BAn/AbAn *100 = Liquid Compositions, Plagioclase Compositions and Tie Lines Along the boundary curve separating the field of Di + L from Plss + L, two solid phases (Di and Plss) coexist with a single liquid. These three phases can be joined together with three tie lines: 1. DiY 2. DiX 3. XY, where o o o Di represents the composition of diopside Y represents the composition of the plagioclase in equilibrium with liquid X and solid Di; X represents the composition of the liquid in equilibrium with Solid Di and PlY. Note that the apex of this small triangle which lies on the boundary curve represents the liquid composition and points down the boundary curve, in the direction of decreasing temperature. A specific liquid composition on the boundary curve coexists only with a plagioclase of specific composition; e.g. liquid X coexists with Di and Pl of composition Y. The tie line joining PlY with the liquid at X must be determined experimentally, it is not obvious from simply examining the diagram. Diopside of fixed composition can coexist with any composition of plagioclase. This is readily apparent from examining the phase diagram. We can draw a series of tie lines (Click Here) which radiate from the Di apex to the Ab-An join, spanning the range of plagioclase compositions from 100% Ab - 0% An to 0% Ab - 100% An. EQUILIBRIUM CRYSTALLIZATION 1 Composition A For an initial liquid of composition A the final solid after Equilibrium crystallization must be a mixture of Di and plagioclase of composition B (PlB), in the proportions determined above. The path followed by the liquid is shown in blue, with the sequence of events as follows: 1. Above the liquidus surface we have 100% liquid. 2. Cool this liquid to the liquidus 3. At the liquidus, Di begins to crystallize and is in equilibrium with liquid. The liquid composition moves directly away from Di towards the boundary curve. 4. From A to C, Di contiunes to crystallize and the liquid moves along the path, constantly changing composition to remain in equilibrium with the Di forming. 5. At Point C, on the boundary curve Pl begins to crystallize along with the Di and these two solid phases are in equilibrium with Liquid. 1. The composition of the Di is fixed. 2. The composition of the Pl, represented by Point D, will have an An content greater than B (the final Pl composition in equilibrium with Di). The actual plagioclase composition, i.e. the position of Point D between Ab and An, forming here must be determined experimentally. However, intuitively, we know from our examination of the Ab-An binary system that at equilibrium, the liquid is always more Ab-rich than the coexisting Pl, so the plagioclase must lie on the An side of the final Pl composition. 3. The composition of the Liquid is represented by Point C. Note that the three points above, Di, C and D, form a triangle (the purple lines). The bulk composition A, lies on or very close to the Di-C join, which lies directly opposite D (the Plagioclase apex), giving <<< 1% Pl in the proportions of the three phases which coexist for composition A at Point C. 6. As cooling continues, the liquid composition moves down the boundary curve from C to E. 7. At E, in equilibrium we have Di, Pl, represented by point F, and Liquid represented by point E. These three phases are linked by the green tie lines. Note that the position of the green triangle has shifted from that of the purple triangle by rotating about the Di apex, common to both, resulting in the shifting of both the liquid composition, from C to E, and the plagioclase composition, from D to F, to more Ab-rich/An-poor compositions. 8. As cooling continues, the liquid composition moves down the boundary curve from E to G. 9. At G, in equilibrium we have Di, Pl, represented by point H, and Liquid represented by point G. These three phases are linked by the red tie lines. Note that the position of the red triangle has shifted from that of the purple and green triangles, again by rotating about the Di apex, common to all three, resulting in the shifting of both the liquid composition, from E to G, and the plagioclase composition, from F to H, to more Ab-rich/An-poor compositions. 10. When the plagioclase has reached it's final composition (Point B), the last liquid is consumed. The composition of the last liquid given by I, expressed in terms of the three end member components Di, Ab and An, on the boundary curve. The cyan tie lines join the Di, the last Liquid composition - I, and the final plagioclase composition B. Examining the cyan triangle shows that the initial bulk composition point A now lies along the Di-PlB edge of the triangle, representing <<< 1% liquid, telling us that crystallization has ceased. The final solid mixture present consists of % Di and % plagioclase of composition B. The important things to remember about equilibrium crystallization in this system are: 1. Equilibrium between the plagioclase and the liquid must be maintained as: 1. The liquid migrates down the boundary curve it continuously reacts with the previously formed plagioclase, causing the liquid to become more sodic, i.e. more Ab-rich; 2. While at the same time the liquid continues to precipitate plagioclase with a higher soda content, i.e. more Ab-rich. 2. Tie lines which join liquid compositions along the boundary curve with plagioclase compositions along the Ab-An join must be determined experimentally. EQUILIBRIUM CRYSTALLIZATION 2 Composition X For an intitial liquid of composition X, the final solid after equilibrium must be a mixture of plagioclase of composition N and Di, in the proportions given by applying the lever rule to the line DiN. The path followed by the liquid is shown in blue, with the sequence of events as follows: 1. Above the liquidus surface we have 100% liquid. 2. Cool this liquid to the the liquidus. 3. On cooling to the liquidus, the liquid first crystallizes an An-rich plagioclase, represented by Point T. The actual composition of the plagioclase forming from the liquid can not be worked out by examining the diagram, it must be determined experimentally. 4. As cooling continues the liquid moves down the liquidus surface from X to S, precipitating a more Ab-rich plagioclase, while the previously formed An-rich plagioclase becomes more sodic by reacting with the liquid. This double reaction between the liquid and solid causes the liquid to migrate in a curved path to the boundary curve at S. The actual liquid path must be determined experimentally. 5. At S, on the boundary curve. Di begins to crystallize and is in equilibrium with Pl represented by Point R and a liquid of composition S. To maintain equilibrium the liquid and the plagioclase compositions are constantly changing, such that each successive liquid is more Ab-rich and each new plagioclase grain is more Ab-rich than the previous one. 6. From S to Q to O the liquid composition moves down the boundary curve. Each new liquid is in equilibrium with a new plagioclase of composition R, P and N respectively, linked by appropriate coloured tie lines. The last drop of liquid is used up at O, yielding a solid mixture of Di and Pl of composition N, in the proportions given by X. FRACTIONAL CRYSTALLIZATION Fractional Crystallization For fractional crystallization, early formed plagioclase does not react with the liquid. The result is that the liquid will migrate to a lower point on the boundary curve than for the same bulk composition which has undergone equilibrium crystallization. Examination of plagioclase in igneous rocks indicates that such a process of incomplete reaction is common, as observed in the presence of zoned plagioclase grains which exhibit normal, reverse and oscillatory zoning. For pure fractional crystallization the last liquid would have a composition represented by the binary eutectic composition on the Di-Ab join and would produce a solid mixture of Di and Ab in the proportions given by this binary eutectic METAMORPHISM - INTRODUCTION Metamorphism is defined as the mineralogical, chemical and structural adjustments in solid rocks to physical and chemical conditions which have been imposed due to changes in pressure and temperature or both. Metamorphism occurs below the surface zones of weathering and cementation. Conditions of metamorphism differ from the conditions under which the rocks in question were originally formed. Metamorphism produced as a result of the progressive increase in temperature and pressure, i.e. by burial of a rock within the earth, is termed prograde metamorphism and in general terms is characterized by dehydration reactions, which release water. With increasing depth of burial the pressure and temperature of the material increases along the follwong gradients: P gradient 3.5 kbar/10 km T gradient 20-30°C/km These gradients cause dehydration reactions to be driven to the right, where water vapour is a producrt of the reaction. e.g. Mineral (OH)x Mineral + H2O Muscovite + Quartz ---> Al2SiO5 + alkali feldspar + water vapour Muscovite ---> alkali feldspar + corundum + water vapour TEMPERATURE LIMITS OF METAMORPHISM The lower temperature limit, at metamorphism takes place, marks the boundary between metamorphism and diagenesis. Lower T = 100-150°C - excludes any diagenetic changes. Some workers consider any changes not occurring under atmospheric conditions to be the result of metamorphism. High T - upper boundary at 700-900°C, over a wide pressure range, with excess water, depending on bulk composition of the rock. Generally considered to end when large scale melts of igneous character are produced. TERMS When examining an individual thin section of a metamorphic rock, two groupings of minerals are present mineral assemblages and mineral associations, are present. The mineral assemblage or paragenesis consists of those minerals which grew at the same time in response to the metamorphism, i.e., they are in equilibrium, and exhibit a stable or metastable coexistence during the metamorphic event of interest. The minerals are in contact with each other. In contrast the mineral association is a list of all minerals observed within a single thin section, whether they exist in equilibrium with each other or not. Minerals in the association may exist metastably in the rock being examined. Any relict grains from the original protolith would fall into the association. A given mineral paragenesis forms under specific pressure and temperature conditions which reflect the metamorphic grade. Metamorphic Grade - is used to signify the degree or state of metamorphism, which approximately corresponds to a progressive increase in temperature. Four Divisions of Grade are recognized: Very low grade Low grade Medium grade High grade The boundaries between the four divisions of grade are marked by significant changes of mineral assemblages in common rocks, corresponding with specific mineral reactions. e.g., The boundary between medium grade and high grade, is based on two specific reactions: 1. Takes place at P< 3.5 kbars The reaction that defines this boundary is: Muscovite + Quartz = Orthoclase + Al2SiO5 + H2O vapour o o T = 580°C P = 1 kbar T = 600°C P = 3 kbar 2. At P > 3.5 kbars This boundary is defined by the reaction: Albite + Orthoclase + Quartz = Granitic Liquid corresponds to the water saturated granite solids o T = 660°C P = 3 kbars o T < 600°C P = 6 kbars METAMORPHIC FACIES Set of metamorphic mineral assemblages, repeatedly associated with each other in space and time. There is a predictable and common correspondence between the mineralogy of each rock and its bulk chemical composition. The mineral assemblage defining the metamorphic facies indicates that a state of stable equilibrium has been attained over a restricted P-T condition. Several different facies have been formally recognized and have names referring to distinct aspects of the facies. e.g. green schist facies = characterized by schists in which green chlorite, talc, serpentine, epidote and actinolite are predominant. Mineralogical characteristics of the metamorphic facies corresponding to those on the Handout - Facies vs. Grade are: 1. Zeolite Facies o Zeolites are groups of white to colourless hydrous alumina silicates, analogous in composition to feldspars. o Laumontite, heulandite and analcine are common, the assemblage Quartz + Laumontite + Chlorite is diagnostic. 2. Prehnite - Pumpellyite o Prehnite +/- Pumpellyite + Quartz is typical. o Prehnite - Ca2Al2Si3O10(OH)2 Pumpellyite Ca2MgAl2(SiO4)(Si2O7)(OH)2H2O 3. Glaucophane - Lawsonite Schist or Blueschist o High P Low T o Glaucophane (Na2MgAl2Si8O22(OH)2) (alkali-rich amphibole) + Lawsonite (CaAl2Si2O7(OH)2H2O) are characteristic also o Jadiete (NaAlSi2O6) + Qtz + Aragonite 4. Greenschist o Albite + Epidote + Actinolite + Chlorite + Calcite in mafic rocks o pyrophyllite in pelitic rocks 5. Amphibolite o Hornblende + Plagioclase (An > 20) o Kyanite (Al2SiO5) in pelites 6. Granulite o lower crustal lithology o cpx + opx + plag + Fe - Mg garnet 7. Eclogite o lower crust or upper mantle lithology o Feldspar-free assemblages, with jadiete-rich cpx + pyrope (Mg-rich garnet) The pressure and temperature range for each facies given in Handout - Facies vs. Grade. The assemblages listed above are mainly for mafic (Metabasite) lithologies. Boundaries between individual facies are not sharp, marked by reactions which occur over a P-T range depending on composition. TYPES OF METAMORPHISM 1. Regional Metamorphism o o o Corresponds to progressive changes, increases, in Temperature & Pressure, due to burial. Commonly this type of metamorphism is associated with orogenic events and processes. Regionally metamorphosed rocks occur in very large belts, 10 - 100's km wide by 100 - 1000's km long. Within each belt will be preserved at least one thermal axis, representing the maximum temperature of metamorphism within the belt. Temperatures will increase towards the thermal axis. Associated granitic and/or ultramafic masses intruded into the terranes generally, but not always near the thermal axis. 2. Contact Metamorphism o o Recrystallization, production of metamorphic mineral assemblages, within the contact aureole surrounding an intrusive body Generally held that P remains constant and metamorphism results from temperature increase, due to conduction of heat away from intrusive. 3. Pyro Metamorphism o o Recrystallization at high temperature of xenolith fragments in volcanic rocks or small intrusions; Melting of the xenolith may occur. 1. 2. Hydrothermal Metamorphism o Recrystallization associated with and influenced by a hydrothermal solution. 3. Cataclastic Metamorphism o Crushing or grinding of rocks as a result of fault movement. COMPOSITIONAL GROUPS In the lab section of the course we will spend two lab sessions examining pelites and basites. 1. Pelites - derived from pelitic sediments (mudstones) - characterised by high Al2O3 & K2O contents, which result in abundant micas produced during metamorphism o o Muscovite - common in low temperature pelites Biotite - common in higher temperature pelites Pelites often display a well developed schistosity, defined by parallel alignment of micas - Micaschists Also present in pelite one will have minerals rich in Al, e.g., o o o o Al2SiO5, kyanite, andalusite, sillimanite Staurolite Fe2Al9O6(SiO4)4(OH)2 Cordierite Mg2Al3(AlSi)5O18 Garnet (Fe,Mg)3Al2(SiO4)3 2. Basic Rocks (Metabasites) Equivalent to basaltic rocks with 50 wt % SiO2. Basalts are often included in sedimentary piles within orogenic belts, thus mineral assemblages in metabasites can be correlated with assemblages in associated pelites as both formed under similar pressure and temperature conditions. Basic rocks rich in MgO, FeO, CaO, & Al2O3, metamorphic minerals produced include: - Chlorite, actinolite, epidote, at low temp greenschist - Hornblende, plagioclase in amphibolite - Hornblende, plag & garnet, opx & cpx in granulite BARROVIAN METAMORPHISM - INTRODUCTION George Barrow mapped a widespread series of zones of progressive metamorphism, as observed in the Dalradian sequence exposed in Scottish Highlands, in the late 19th century. Each zone recognized is based on the first appearance of a group of distinctive index minerals as the highest metamorphic grade, along the thermal axis, was approached. Thermal axis has abundant granitic bodies situated along its length. Index mineral is one which is characteristic of that zone - any mineral within the zone may be the index mineral. The Dalradian sequence consists of Precambrian and early Cambrian sediments, overlain by unmetamorphosed Upper Devonian Sandstones. Within the Dalradian sequence, Barrow recognized the existence of unmetamorphosed shaley (pelitic) sediments which subsequently underwent metamorphism. BARROW'S ZONES The work carried out by Barrow led to his recognizing the following metamorphic zones within the Dalradian Sequence: 1. Zone of digested clastic mica o o Now termed chlorite zone - characterized by first appearance of chlorite. The characteristic assemblage - quartz-chlorite-muscovite-albite. 2. Biotite Zone Marked by the first appearance of red-brown biotite produced from reaction between muscovite and chlorite. 3. Garnet Zone 1. The characteristic assemblage recognized by Barrow is quartz-muscovitebiotite-almandine (Fe,Mg)3Al2Si3O12-albite or oligoclase. 2. Staurolite Zone The assemblage is quartz-muscovite-biotite-almandine-staurolite-(oligoclase) 3. Kyanite Zone 1. Assemblage is quartz-biotite-muscovite-oligoclase-almandine-kyanite 2. Sillimanite Zone quartz-biotite-muscovite-oligoclase-almandine-sillimanite Zones were mapped in a small portion of Aberdeenshire and latter extended across the full extent of the Highlands. Some minerals, e.g., biotite, continue through the higher grade zones, however others, e.g., staurolite, disappear in the next zone INTERPRETATION OF BARROW'S ZONES Barrow believed the zones resulted from the heat from the small granitic intrusives found in the high grade zones - Contact Metamorphism Another geologist, (C.E. Tilley) working on the same rocks in a different area suggested that the temperature of each zone was largely determined by the depth of burial (geothermal gradient), modified at depth by heat from the intruded granites. P - T Grid for Dalradian Sequence Tilley (1924) suggested that the isograds mark rocks originating under closely similar physical conditions of temperature and pressure - not greatly different from what we believe today. The first appearance of an index or zone mineral indicates a definite metamorphic grade, as long as the rocks were of an appropriate composition for that mineral to grow. The appearance of a particular mineral depends on the following variables: 1. Conditions under which the metamorphism occurred, i.e., temperature, water pressure, load pressure. 2. Rates of nucleation and reaction involved in formation of the mineral. 3. Composition of the rock. The last variable is the most important factor in the production of a given mineral under different metamorphic conditions. An isograd mapped within a single, homogeneous layer closely approaches the idealized concept of a line of equal grade. Correlation of an isograd from one area with one mapped in a widely separated area, can in rocks of similar composition be hazardous as each rock may have formed under different conditions at different times. A Finnish geologist, Eskola, was the first to attempt to correlate metamorphic zones between widely separated areas. Working in Finland, Eskola found that contact metamorphic assemblages were related to grade and composition. When he compared his results to others he found that some mineral assemblages were the same between areas, while in other areas in rocks of similar composition the assemblages were quite different. Led Eskola to calculate that rocks from the two different areas were metamorphosed under different conditions, e.g., P & T and prompted him to propose a classification of metamorphic rocks - an association of metamorphic rocks, each consisting of a mineral assemblage consistently related to the composition of the rock - Facies. Handout shows the relationship between metamorphic zones and metamorphic facies. Not only for pelitic rocks as mapped by Barrow, but also for Basic and Calcerous rocks. If all the rock types in the table were interbedded correlation of the mineral assemblages within each, rock type would be very simple. Such a correlation, for several rock types within a single metamorphic grade, defines the metamorphic facies for that grade.