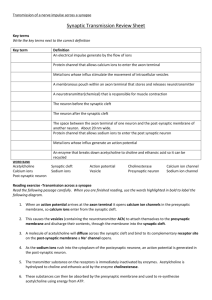
Physiology Lecture Outline: Membrane Potential and Neurophysiology
advertisement
Physiology Lecture Outline: Membrane Potential and Neurophysiology
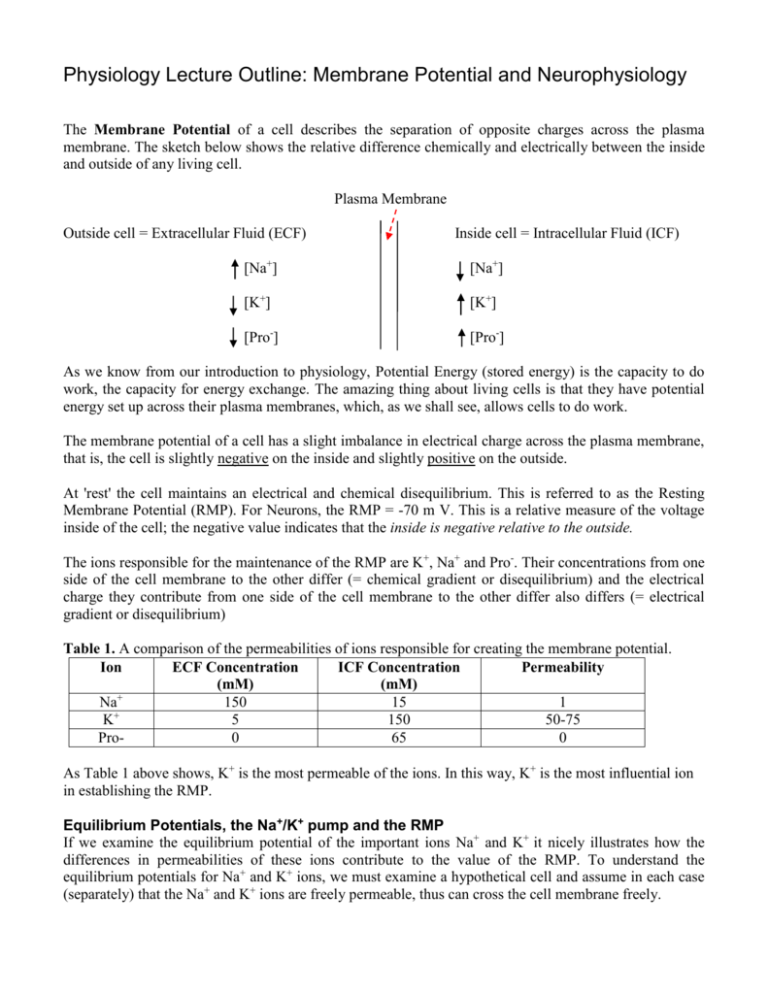
The Membrane Potential of a cell describes the separation of opposite charges across the plasma
membrane. The sketch below shows the relative difference chemically and electrically between the inside
and outside of any living cell.
Plasma Membrane
Outside cell = Extracellular Fluid (ECF)
Inside cell = Intracellular Fluid (ICF)
[Na+]
[Na+]
[K+]
[K+]
[Pro-]
[Pro-]
As we know from our introduction to physiology, Potential Energy (stored energy) is the capacity to do
work, the capacity for energy exchange. The amazing thing about living cells is that they have potential
energy set up across their plasma membranes, which, as we shall see, allows cells to do work.
The membrane potential of a cell has a slight imbalance in electrical charge across the plasma membrane,
that is, the cell is slightly negative on the inside and slightly positive on the outside.
At 'rest' the cell maintains an electrical and chemical disequilibrium. This is referred to as the Resting
Membrane Potential (RMP). For Neurons, the RMP = -70 m V. This is a relative measure of the voltage
inside of the cell; the negative value indicates that the inside is negative relative to the outside.
The ions responsible for the maintenance of the RMP are K+, Na+ and Pro-. Their concentrations from one
side of the cell membrane to the other differ (= chemical gradient or disequilibrium) and the electrical
charge they contribute from one side of the cell membrane to the other differ also differs (= electrical
gradient or disequilibrium)
Table 1. A comparison of the permeabilities of ions responsible for creating the membrane potential.
Ion
ECF Concentration
ICF Concentration
Permeability
(mM)
(mM)
Na+
150
15
1
+
K
5
150
50-75
Pro0
65
0
As Table 1 above shows, K+ is the most permeable of the ions. In this way, K+ is the most influential ion
in establishing the RMP.
Equilibrium Potentials, the Na+/K+ pump and the RMP
If we examine the equilibrium potential of the important ions Na+ and K+ it nicely illustrates how the
differences in permeabilities of these ions contribute to the value of the RMP. To understand the
equilibrium potentials for Na+ and K+ ions, we must examine a hypothetical cell and assume in each case
(separately) that the Na+ and K+ ions are freely permeable, thus can cross the cell membrane freely.
2
1) The Movement of Na+ ions alone:
If it is assumed that Na+ ions are freely permeable, with no restrictions to its movement, then Na+ ions
will move back and forth across the membrane until the Electrochemical Gradient has Equilibrated. The
value of the voltage across the membrane for the Equilibrium Potential of Na+ = +60 mV (ENa+ = +60mV)
2) The movement of K+ ions alone:
If it is assumed that K+ ions are freely permeable, with no restrictions to its movement, then K+ ions will
move back and forth across the membrane until the Electrochemical Gradient has Equilibrated. The value
of the voltage across the membrane for the Equilibrium Potential of K+ = -90 mV (EK+ = -90m V)
If these ions were both equally permeable, then the RMP would be somewhere in between these two
values (in between -90 and +60 mV). However, K+ ions are 50 to 75 times more permeable than Na+ and
therefore the RMP is much closer to the EK+ than the ENa+. The value of -70 mV is much closer to -90mV
than to +60 mV.
3) The Na+/ K+ Pump (also called the Na+/K+ ATPase):
A transport membrane spanning protein embedded in the plasma membrane that 'pumps' Na+ and K+ ions
across the membrane against their concentration gradients. To do this, it requires ATP directly, and so it is
a primary active transport mechanism. It pumps out or ejects 3 Na+ ions from the inside of the cell and
pumps in or imports 2K+ into the cell from the outside at the cost of 1 ATP for one cycle of the Na +/K+
pump. The pump is a protein that has catalytic ability (is an enzyme as well) and hydrolyzes ATP to ADP
+ Pi and heat.
Both Na+ and K+ ions continuously "leak" across the cell membrane down their concentration gradients
(through open protein channels or ‘pores’ in the membrane). Because of this, the Na+/ K+ pump must be
active all the time in order to constantly bailout the leaky ship and maintain the RMP. In summary, it is
these three issues that contribute to the maintenance of the RMP.
There are 4 types of primary tissues in the body:
1. Epithelium
2. Connective
3. Muscle *
4. Nervous*
*excitable tissue (responds to electrical stimulation).
The excitable tissues have various RMP's, for example; neurons have a RMP of -70mV whereas most
cardiac muscle cells have a RMP of -90mV. Excitable means that they are capable of producing electrical
signals when excited (stimulated). As we may already know, the flow of charged particles is an electrical
current, and these currents are used to send signals or do work.
Neurons and the Nervous System (NS)
Neurons are the cell of communication in the NS, so we need to know just a little about its basic anatomy.
Label this generalized neuron and indicate briefly what important functions occur at the various locations.
3
There are two ways that a neuron can undergo rapid changes in RMP and this really means that there are
two ways that neurons can electrically communicate. These are Graded Potentials and Action Potentials.
Graded Potential = a local change in membrane potential with varying degrees of magnitude. For short
distance communication. The stronger the triggering event, the stronger the graded potential.
What is a trigger? Here are some examples of what can trigger a graded potential:
1. A Specific Stimulus - a change in temperature, pH, light intensity, etc.
2. A Surface Receptor on plasma membrane - binding of the receptor by a ligand.
3. Spontaneous change in membrane potential - may be caused by 'leaky' channels, etc.
The spread of a graded potential is decremental - that is, it diminishes over distance.
Action Potential = a brief reversal of resting membrane potential by a rapid change in plasma membrane
permeability. 'Reversal' => from -70mV to +30mV back to -90mV. For long distance signal transmission.
The spread of an action potential is non-decremental, that is, the strength of the signal does not diminish
over distance, and it is maintained from the site of origin to destination. An action potential can be
described as an All or None event. During an action potential, significant changes occur in membrane
permeability for Na+ and K+. This causes rapid fluxes of theses ions down their electrochemical gradients.
There are 4 main phases of an action potential:
1. Threshold
2. Depolarization phase
3. Repolarization phase
4. Hyperpolarization phase
For an action potential to occur, threshold must be reached. The threshold value in neurons is -55 mV.
When the RMP is altered and it reaches threshold, this change in the voltage of the membrane causes
voltage gated Na+ channels to open, and this triggers the onset of an action potential.
Described below is the general sequence of an action potential, but before that, it is helpful to recognize
the various types of gated ions channels in the plasma membrane of neurons.
There are three types of Gated Ion Channels
1. Voltage Gated - channel opens and closes in response to changes in membrane potential of cell.
2. Ligand (chemically) Gated - channels open and close in response to binding of a specific chemical
messenger with a membrane receptor in close association with a channel. Conformational changes occur
due to ligand -receptor complex.
3. Mechanically Gated - activation of channel from mechanical distention of cell membrane, there is a
stretch or deformation of the plasma membrane causing the channel to open.
The Positive Feedback Loop of voltage gated Na+ ion channels.
The triggering event at depolarization increases the membrane voltage (it becomes more positive), which
opens voltage gated Na+ channels, causing the influx of Na+. This influx further increases the membrane
4
+
+
voltage, leading to the opening of more voltage gated Na channels, causing greater influx of Na further
increasing the voltage . . . and on and on, in other words, this is an example of a positive feedback loop.
The loop is broken at the voltage of +30mV, at this point the voltage gated Na+ channels close and are
unable to open again (become deactivated). These channels typically cannot open again until RMP has
been restored (-70mV). The nature of this voltage gated Na+ channel is important in creating the absolute
refractory period. Below are shown the three conformational (shape) states of the voltage gated Na+ ion
channels.
1. Closed
(able to open)
2. Open
3. Closed (deactivated)
(unable to open)
The General Sequence Events of an Action Potential
The result of the opening of voltage gated Na+ channels when threshold is reached (and the positive
feedback loop that ensues) is that Na+ floods into cell and the inside of the cell becomes more positive
very quickly, going from -55 mV (resting) towards a positive value of +30 mV. Recall that the ENa+ = +60
mV, therefore the membrane is getting closer to this value. At the 'Peak' of the action potential (+30mV),
the Na+ channels close (become deactivated) and remain closed and inactive until RMP is restored.
All the while, the slow to open K+ channels continue to open and at the peak of the action potential K+
rush out of the cell, down their concentration gradient. This outward movement of K+ starts to restore
membrane potential back toward RMP (the membrane voltage is decreasing now but the potential is
increasing). This is the Repolarization phase; the cell is becoming more negative inside as the positively
charged K+ leaves the cell.
These K+ channels are also slow to close and continue to allow the positively charged K+ to leave the cell.
This leads to a more negatively charged cell inside and represents the Hyperpolarization phase of the
action potential. As the slow closing K+ finally close, the resting permeability of the cell is restored, RMP
is restored and the action potential is over.
An Action Potentials has 2 Refractory Periods
1. Absolute Refractory Period: During this period, the cell is unresponsive to any further stimuli. No
other action potential can be fired at this point, regardless of the strength of the stimuli.
The role of the Absolute refractory period is to ensure one-way propagation of action potentials.
2. Relative Refractory Period: During this period, another action potential can be produced but the
strength of the stimuli must be greater than normal to trigger an action potential.
The role of the Relative refractory period: helps to limit the frequency of action potentials.
Summation
Summation is when the magnitude of graded potentials can be added together, to have a combined effect
on the postsynaptic membrane. Summation of graded potentials can occur in two ways: Temporal
Summation and Spatial Summation.
5
Temporal Summation occurs from the summation of graded potentials overlapping in time. In other
words (using the example in class), as the frequency of signals (action potentials) from neuron A to
another neuron, (neuron X) increases, the graded potentials (from A) can summate.
Spatial Summation occurs from the summation of several graded potentials from several converging
neurons simultaneously. In other words (again using the example in class), when several different neurons
in space (e.g., A and B) send a signal simultaneously to neuron X, these graded potentials that are sent at
the same time are summated by neuron X.
Comparison of Graded and Action Potentials
Below is a side-by-side comparison of graded and action potentials.
Graded Potentials
Action Potentials
1) Magnitude varies
1) No variation - All or None
2) Decremental (passive spread)
2) Non-decremental (self-regenerating)
3) No Refractory Periods
3) Two Refractory Periods (absolute and relative)
4) Summation is possible
4) No Summation possible
5) Trigger: NT's, hormones, etc.
5) Trigger: Threshold reached
6) Occurs at cell body (direction can vary)
6) Occurs at axon hillock (one way direction)
Speed of the Conduction of the Signal
Although the magnitude of an action potential is always the same, the speed of the propagation of an
action potential down an axon can vary.
1. Diameter of Axon
Compare the cross sectional diameter of axons A and B.
Which of these axons will conduct a signal faster and why?
A
B
The larger axon will conduct a signal faster than a smaller axon. This is because there is less friction
between the moving charged particles (Na+ and K+) and the sides of the axon in the larger axon. Axons in
the human body do vary in their diameter, but there is a limit to how large the diameter of an axon can be
within the confines of the entire human body.
2. Temperature
When the surrounding temperature increases, chemical reactions speed up. Thus, if axon temperatures
increase, the rate of conduction of the impulse down the axon will increase. Conversely, if temperatures
decrease, the rate of conduction of the impulse down the axon will also decrease. Normally, body
temperature remains very constant but can change dramatically in some situations. Typically a dramatic
6
drop in Tb will significantly slow down neuronal transmission. For example, if a person falls into the very
cold water of a frozen over lake, all of their nervous responses will be significantly slowed.
3. Myelination of Axon
The myelin sheath that covers some axon is made from the cytoplasm of glial cells (Schwann cells in the
PNS and oligodendrocytes in the CNS). The myelin sheath is mostly composed of lipids and therefore is a
good insulator, which is the same as saying it is a poor conductor of electrical charge. In this way, it
reduces the electrical 'leakiness' along the axon and helps to conduct the signal more quickly.
Little gaps in the myelin sheath, called 'Nodes of Ranvier', allow the action potential to move faster along
the axon. The electrical signal is said to jump from node to node, thus it is called Saltatory Conduction.
This is not what actually happens at the Nodes of Ranvier, but at this stage it is convenient to think of the
signal 'jumping' down the myelinated axon significantly faster than a non-myelinated axon.
Of these three factors that can effect the speed of an action potential traveling down an axon, (diameter,
temperature and myelination), it is axon myelination that is the most significant. This is mainly because
axon diameter and body temperature are kept fairly constant.
The degenerative disease multiple sclerosis is due to the destruction of the myelin sheath on somatic
motor neurons that control skeletal muscle movement. Initially it causes a slowing of the signal and
eventually it can stop motor signals to skeletal muscle all together. The sensory neurons that are bringing
in sensory information are not affected by multiple sclerosis. So, you could feel your legs normally but
would have problems sending signals out for muscle control.
Synaptic Transmission - The Sequence of Events
A synapse is the site of communication between two neurons. Draw and label the pre- and post-synaptic
neurons of a synapse. Include ion channels, vesicles, receptors and enzymes.
Events in the Pre-Synaptic Neuron
1. A nerve impulse or action potential (AP) moves down an axon and arrives at the synaptic terminal.
2. Voltage gated Ca2+ ion channels open in response to the change in membrane potential from the AP.
3. The concentration gradient favors an influx of Ca2+ ions from the extracellular fluid into the cell.
4. This increase in intracellular Ca2+ ions ([Ca2+]i) triggers exocytosis of the synaptic vesicles that are
'docked' on the membrane.
5. The vesicles release their neurotransmitter (e.g., ACh, NE, Dopamine, Serotonin, etc.). After
neurotransmitter (NT) is released, the empty vesicles drop back into synaptic knob and may reload
with more NT. The increase in [Ca2+]i also causes more vesicles to detach from cytoskeleton and
dock with membrane in preparation for the next release of NT.
6. The NT is released by exocytosis and crosses the synaptic cleft by simple diffusion to reach the
receptors on the postsynaptic membrane.
Events in the Post-Synaptic Neuron
7. The NT released from pre-synaptic neurons binds to receptors on the postsynaptic membrane.
7
8. Some post-synaptic membrane receptors can act as ligand (chemically) gated ion channels, that is,
they open in response to being bound by signal molecules. For example, many ligand gated channels
allow both Na+ and K+ to diffuse down their concentration gradients. Others allow CI- ions to travel
down its concentration gradient.
9. If we use a ligand gated Na+ ion channels as an example, when the ligand gated Na+ ion channels
open, Na+ diffuses along the inner surface of the post-synaptic neuron, this influx of Na+ partially
depolarizes the membrane, creating a local PostSynaptic Potential (PSP).
10. Response of the postsynaptic neuron?
If the membrane potential is depolarized and brought closer or to threshold, then it is called an
Excitatory PostSynaptic Potential (EPSP). For example, if Na+ ions enter the cell - the inside of
the cell becomes more positive, and the RMP of -70 mV gets moved closer to threshold (-55 mV).
If the membrane potential is hyperpolarized and moved further away from threshold, then it is
called an Inhibitory PostSynaptic Potential (IPSP). For example, if K+ ions leave or CI- ions enter
the cell, the inside becomes more negative, and the RMP of -70 mV gets moved further away from
threshold, making the cell less likely to reach threshold.
lonotropic and Metabotropic Effects
Ionotropic Effects - The mechanisms described above are termed ionotropic effects, whereby a
neurotransmitter (NT) binds to a membrane receptor and directly opens an ion channel. This then leads to
a rapid change in membrane potential of postsynaptic cell, whether Excitatory or Inhibitory. This type of
effect is very common for Nervous system transmissions, which are rapid and brief.
Metabotropic Effects - The mechanisms of metabotropic effects are mediated by a second messenger
system, like cAMP.
1. Presynaptic neuron releases NT (first messenger) via exocytosis into synaptic cleft.
2. The NT diffuses across synaptic cleft and binds receptors on postsynaptic membrane of neuron.
3. The receptor is linked to and activates a G protein which hydrolyses GTP to GDP. This allows a
subunit to migrate along plasma membrane to the inactive enzyme adenylyl cyclase.
4. The G protein subunit activates adenylyl cyclase (an enzyme which uses ATP as its substrate).
5. Adenylyl cyclase removes 2 phosphate groups from ATP to make cyclic AMP (cAMP) - this is the
cell’s second messenger (this form of cell communication is called “the second messenger system”).
6. The increase in cAMP inside the cell activates a Protein kinase (e.g., PKA).
7. A protein kinase phosphorylates (adds phosphates to) other enzymes or other protein structures in
the cytosol and can alter activity of that structure (that is, can increase or decrease its activity).
The sequence of events above can have several effects
1. Activated enzymes trigger genetic transcriptions and synthesis of new proteins.
2. Activated enzymes activate other metabolic pathways.
8
3. Activated enzymes open ligand gated channels in plasma membrane.
Stopping Signal Transmission
A number of things must occur to stop the postsynaptic cell from responding and begin to restore cell to
resting state so that it can receive and possibly transmit a signal again.
Important things must happen
1. Stop Impulse: The impulses from presynaptic nerve fiber stops. The action potential ends, and no
further release of NT into synaptic cleft occurs.
2. Clear Synaptic Cleft: The synaptic cleft must be cleared of residual neurotransmitter (NT), in
preparation of another signal arriving. This can be achieved in 3 ways:
1) Diffusion of NT away from receptors in the synaptic cleft.
2) NT reuptake by presynaptic neuron. Recycling can be of the entire NT or in fragments.
3) Degradation of NT enzymatically. This hastens the return of the membrane pot to RMP.
e.g. ACh + Acetalcholinesterase produces Acetate + Choline (both are non-stimulating fragments).
e.g., NE, E, Serotonin + MonoAmine Oxidase produces non-stimulating fragments of these NT.
Neurotransmitters
Neurotransmitters are signal molecules that are released from neurons. There are believed to be about 60
known neurotransmitters. They can function as excitatory (EPSP) or inhibitory (IPSP) substances, but this
can change depending on the location of neuron and type of effector (target) cell it acts on. For example,
Acetylcholine (ACh) contracts skeletal muscle and ACh relaxes smooth muscle! How can the same NT
have contrasting effects on various tissues? The answers lies in the type of receptor on the target tissue.
The specific type of receptor on the tissue will determine how the tissue responds to various signal
molecules.
Neurotransmitters can be divided into four categories:
Acetylcholine (ACh), Amino Acids, Biogenic Amines and Neuropeptides
ACh - This is a single molecule that is in a class all by itself. Neurons that release ACh are termed
"Cholinergic Neurons". It is best known in neuromuscular junctions (NMJ) effecting skeletal muscle. It
is released by many neurons in the peripheral nervous system (PNS) and some neurons in the central
nervous system (CNS). ACh binds to two types of receptors, 1) nicotinic and 2) muscarinic.
In the PNS, ACh is the sole NT used by the Somatic nervous system (SNS): Here at the NMJ ACh binds
to nicotinic receptors on skeletal muscle and causes excitation (contraction) of skeletal muscle. In the
Autonomic nervous system (ANS), it is release by all neurons at the ganglia and binds to nicotinic
receptors on postgalionic neurons. It is also released by parasympathetic postgalionic neurons and binds
with muscarinic receptors on effector tissue (cardiac muscle, smooth muscle and glands). In general
terms, nicotinic receptors are always excitatory (in that when stimulated they cause an EPSP) and
muscarinic receptors are generally inhibitory (in that when stimulated they usually cause an IPSP).
Amino Acids - These NT's can be excitatory or inhibitory.
9
A) Excitatory
1) Glutamate - accounts for approximately 75% of all excitatory transmission in the brain, so it is the
most common excitatory NT in the brain. It is released in cerebral cortex, brain stem. Involved in learning
and memory. Also called glutamic acid.
2) Aspartate - similar to glutamate but found mostly in the spinal cord for excitation. (aspartic acid)
B) Inhibitory
3) GABA - Gamma AminoButyric Acid (GABA) is the most common inhibitory NT in the brain.
Released in thalamus, hypothalamus, cerebellum, occipital lobe and retina.
4) Glycine - is the simplest amino acid and is the most common inhibitory NT in the spinal cord. It is also
released in the brain and retina.
Biogenic Amines - These NT's are all derived from either the amino acid tyrosine or tryptophan.
The COOH groups in the amino acid are replaced by NH2 groups. There are two main categories of
Biogenic Amines, they are A) Catecholamines (derived from tyrosine) and B) Indolamines (derived from
tryptophan). All of these can also be referred to as monoamines, which are degraded by the enzyme
MonoAmine Oxidase (MAO).
A) Catecholamines - three main catecholamines: Epinephrine (E), Norepinephrine (NE) and Dopamine.
1) Norepinephrine (NE) - released by most sympathetic postganglionic nerve fibers. Also released in the
cerebral cortex, hypothalamus, brain stem, cerebellum and spinal cord. Has a role in mood, dreaming,
wake and alertness levels. Neurons that release NE or E are termed "Adrenergic Neurons". For the most
part, NE is an excitatory or stimulatory NT, typically elevating mood and alertness.
* The highly addictive drug cocaine interferes with NE transmission in the brain. Cocaine acts to block
the reuptake of NE back into adrenergic neurons that released it. This has an effect of increasing the
amount of NE that lingers in the synaptic cleft, thus increasing the stimulatory effects on the target cell.
* There are also drugs that inhibit the effects of the degradative enzyme Monoamine Oxidase (MAO),
they are called Monoamine Oxidase Inhibitors (MAO Inhibitors). They have their effect by increasing the
amount of NE that remains in the synaptic cleft, as well as increasing the amount of NE that is packaged
into the vesicle before being released into the synaptic cleft. Some medications act to reduce the amount
biogenic amine action in the body and are used for high blood pressure (e.g., reserpine) but can have the
side effect of causing depression. This is because decreased (or depressed) levels of biogenic amines in
neural transmission is linked to clinical depression.
2) Epinephrine (E) - released in thalamus, hypothalamus, spinal cord and adrenal medulla. Chemically
and functionally similar to the effects of NE. When released from the adrenal gland, it acts as a hormone.
10
3) Dopamine - released by the cerebral cortex, hypothalamus, limbic system and retina. Highly
concentrated in the substancia nigra of the midbrain where it is involved with voluntary motor control.
Also involved in elevation of mood and emotional responses. Neurons that release dopamine are termed
"Dopaminergic Neurons".
* Dopaminergic neurons in the subsancia nigra normally inhibit primary motor neurons (which then
control skeletal muscle fibers). Degeneration of dopaminergic neurons in the subsancia nigra can lead to
Parkinson's disease. L-Dopa is a precursor to dopamine and used as a medication for Parkinson's disease,
as it can pass through the blood brain barrier, whereas dopamine cannot.
* It has also been postulated that the consumption of chocolate increases dopamine transmission, thus
may lead to feeling good. Dopamine transmission has also been linked to reward centers in the brain (like
the ‘pleasure’ center) and has been associated with addictive behavior.
B) lndolamines - two main indolamines: Serotonin (5-HT) and Histamine.
4) Serotonin (5-HT) - released in the hypothalamus, limbic system, cerebellum, retina and spinal cord.
Also secreted by platelet cells and intestinal cells. Believed to playa role in sleepiness, alertness, mood
and thermoregulation.
*Compare to Melatonin, the hormone released by the pineal gland for inducing sleep (regulating circadian
rhythm).
*Serotonin is also affected by MAO Inhibitors. For example, the drugs phenelzine (Nardil) and
isocarboxazide (Marplan) are also used to treat clinical depression. These also have an effect of increasing
the amount of NE in the synaptic cleft, as well as increasing the amount of NE that is packages into the
vesicle before being released into the synaptic cleft. This elevated NE response tends to be seen in the
sympathetic division of the ANS, so “dry mouth”, elevated heart rate and blood pressure are significant
side effects experienced by people on this type of medication.
* Often MonoAmine Oxidase is located inside the presynaptic neuron where it degrades NT that has been
actively transported back into the cell that released them. In these cells, inhibition of MAO is believed to
increase the amount of serotonin packaged into the vesicle before being released into the synaptic cleft.
Again, this would increase the amount of serotonin released and increase serotonergic effects.
* Some medications prescribed for depression, such as fluoxetine (Prozac) and paroxetine (Paxil),
interfere with serotonin transmission in the brain. They both prevent reuptake of serotonin by presynaptic
neurons. This represents a relatively new class of antidepressants called selective serotonin reuptake
inhibitors (SSRIs). This results in an increased amount of serotonin remaining in the synaptic cleft, thus
serotonin-dependent activity in the CNS increases. These effects are analogous with the effects of cocaine
for NE neurons. The SSRIs are more specific than MAO inhibitors because they only target serotonergic
synapses.
5) Histamine - released by the hypothalamus but little is known about its specific actions as a NT. Also
released by mast cells and basophils. Acts as a paracrine and vasodilates blood vessels.
11
Neuropeptides - These NT's can be from 2 to 40 amino acids in length. There are many
neuropeptide but we will limit our discussion to three: Substance P, Enkephalins and -Endorphins.
1) Substance P - released by neurons of the basal nuclei, midbrain, cerebral cortex and hypothalamus.
This is a very important NT for mediation of pain transmission. The P is for Pain!
2) Enkephalins - released in hypothalamus, limbic system, pituitary gland and pain pathways of the
spinal cord. Also found in nerve endings of the G.I. tract. Enkephalins act as analgesics ('pain killers') by
inhibiting substance P transmission. Levels of enkephalins increase significantly during child birth.
3) -Endorphins - found in many parts of the brain and the G.I. tract, also a hormone in the pituitary
gland. -Endorphins are opioid peptides (similar in chemical nature to opium) and are part of the body's
natural pain relief molecules. This NT also suppresses pain by blocking substance P transmission and
reduces the perception of fatigue.
* Endorphins are released after prolonged physical exertion or during 'stressed' states. It is linked to
'runner's high', the often euphoric feeling experienced by individuals after an endurance run.