Empirical Formula lab - mvhs
advertisement

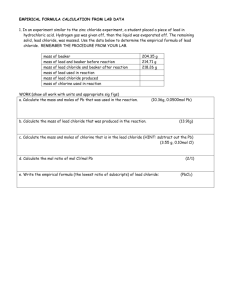
Chemistry Honors Chapter 7 Stoichiometric Determination: Empirical Formula of Copper Chloride Background Empirical Formula: the simplest whole-number ratio in which different kinds of atoms combine to form a compound (atoms combining as single, distinct units). For example: name Empirical Formula Molecular Formula water H2O H2O aluminum chloride AlCl3 AlCl3 ethene CH2 C2H4 glucose CH2O C6H12O6 For ionic compounds: Compound formula is the same as the empirical formula. The compound formula defines the formula unit, the simplest whole-number ratio of positive and negative ions giving an electrically neutral unit. Empirical Formulas and mol: The empirical formula is the simplest whole-number ratio of numbers of moles of atoms in one mole of a compound. The mole (mol) is that quantity of matter possessing a mass equal to the formula weight expressedin grams. For example: Cu (a monatomic element) H2O Al2O3 Atomic Wt.: 63.546 Formula Wt.: 102. Formula Wt.: 18.016 Mass of 1 mol = 63.546 g Mass of 1 mol = 18.016 g Mass of 1 mol = 102. g 63.546 g Cu/mol Cu 18.016 g H2O/mol H2O 102. g Al2O3/mol Al2O3 Empirical formula of Copper Chloride: CuxCly If the compound is made of ... then ... Cl- ions are needed and the formula would be ... Cu1+ and Cl11 CuCl 2+ 1Cu and Cl 2 CuCl2 3+ 1Cu and Cl 3 CuCl3 In this lab you will be producing solid Cu from a Cu(II) solution by reaction with Zn. CuxCly (aq) + Zn (s) ---> ZnCl2 (aq) + Cu (s) (blue) (colorless) Procedure: Chemistry Honors Chapter 7 1. 2. 3. 4. 5. 6. 7. 8. Obtain about 25 mL of .1 M copper chloride solution in a 50 mL graduated cylinder. (Be sure to measure the volume to +/- 0.2 mL.) Transfer to a 100 mL reaction beaker; rinse the graduated cylinder and add the rinse to the beaker. Obtain a piece of Zn and obtain its mass to +/- 0.01 g. Handling the Zn with tongs, add it to the reaction vessel. Carefully tilt beaker so solution covers much of the Zn. Use the rubber policeman attached to your stirring rod to periodically scrape solid Cu from the Zn piece into the solution. Let it react for 10 minutes. After the reaction has stopped, add 5-10 drops of 1.0 M HCl solution and stir. This is to make all the Zn reacts with the Chloride ions. Using tongs, remove the Zn from the beaker, use the policeman to scrape the Zn,making sure to leave behind adhering Cu. Dry the Zn with a paper towel and obtain its mass, then dispose of the Zn in a container under the fume hood. Slowly and carefully decant (pour off) the supernatant liquid from the solid Cu into a waste beaker. Wash the solid Cu by adding about 10 mL distilled water to it, stirring vigorously. After the copper settles, decant the rinse water into your waste beaker. This will remove dissolved ZnCl2. Removing water from the solid Cu Add about 10 mL isopropyl alcohol (isopropanol) to the Cu, stir thoroughly, and decant (gently tip over so that only the liquid goes out leaving the solid behind) the alcohol into the waste beaker. Set up a boiling water bath on a hot plate using a 250-mL beaker almost half full of tap water. You will use this water bath later to dry the Cu. Wipe clean an evaporating dish with your cloth towel. Obtain the mass of the dish to +/- 0.01 g. Use the rubber policeman to transfer the Cu from the reaction beaker to the evaporating dish. Make sure to transfer every trace of solid Cu. You may use a bit of isopropanol to quickly rinse the beaker. Place the evaporating dish on the water bath and heat the water to boiling. When the Cu appears almost dry, stir the Cu and continue heating. When it appears completely dry, use tongs to place the evaporating dish on a wire gauze on the lab bench. Allow the dish and contents to cool to room temperature. Check the cooling of the dish by bringing your hand close to the dish without actually touching the dish. Carefully lift the wire gauze and carry the evaporating dish to the balance. Determine the mass of the dish and its contents to +/- 0.01 g. Using tongs, place the evaporating dish back on the water bath and heat for an additional 5 minutes. As before, remove the dish, let it cool, and weigh it. Repeat the heating, cooling, and weighing until successive weighings are within +/- 0.05 g. Discard the Cu(s) in the waste container. Discard the contents of your waste beaker into the waste container. Chemistry Honors Chapter 7 Calculations 1. How many moles of Zn reacted? 2. How many atoms of Zn reacted? 3. How many moles of Copper Chloride were used? (0.1 M means there is 0.1 mole of copper chloride in every liter of solution) 4. How many grams of copper chloride were used? 5. How many grams of copper were produced? 6. How many grams of chloride ions were used? 7. How many moles of chloride ions? 8. How many moles of copper were produced? 9. Two moles of chloride ions reacted for every mole of zinc ions. How many moles of chloride ions reacted? 10. What is the empirical formula of copper chloride? Write a tentative formula: CuClb/a where a = mols Cu and b = mols Cl. The ratio b/a is the number of moles of Cl needed for every mole of Cu in the compound. If necessary, convert these ratios to whole numbers. For example, if you get ... o ... CuCl0.96, you have approximately CuCl1, that is, CuCl. o ... CuCl0.48, you have Cu2Cl0.96 (because 0.48 moles Cl for every mole of Cu is the same ratio as 0.96 moles of Cl for every 2 moles of Cu), which is approximately Cu2Cl. o ... CuCl1.5, you have Cu2Cl3. Prelab : 1. Drawing a labeled lab set up including the precision of the equipment. 2. A Data table to record your qualitative and quantitative data. Lab Write up will include: 1. Data Processing 2. Error Analysis. Found at website: http://web.lemoyne.edu/~giunta/chm151L/stoichiometry.html