quiz1-0708ans
advertisement

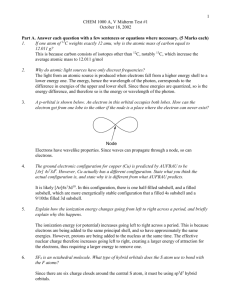
1 CHEM 1000 A, V Midterm Test #1 October 12, 2007 Part A. Answer each question with a few sentences or equations where necessary. (5 Marks each) 1. Which of the following sets of quantum numbers are possible for an electron in a calcium (Ca) atom? (Check one box for each). Quantum Numbers n = 5, l = 0, ml = 0, ms= +½ n = 2, l = 0, ml = 0, ms= +½ n = 3, l = 1, ml = 1, ms= -½ n = 3, l = 2, ml = -2, ms= -½ n = 1, l = 0, ml = 1, ms= +½ 2. Possible Not Possible X X X X X Why does atomic radius decrease going left to right across a period? Electrons are added to the same principal shell, hence are at about the same energy, going left to right in one period. But protons are also being added to the nucleus. These additional protons exert a greater pull on the electrons, which are therefore pulled inwards towards the nucleus, decreasing the radius. This can also be explained in terms of the effective nuclear charge, which increases going left to right. 3. State Hund’s rule. Hund’s rule says that electrons are placed into degenerate orbitals one at a time, and only paired up once each degenerate oribtal contains one electron. This results in the maximum unpairing of electrons in degenerate orbitals. 4. Which molecule contains the more covalent bonds: Cu2O or K2O? How do you know? Cu2O is more covalent. We know this because Cu and O are closer together in the periodic table, hence have electronegativities that are closer than those of K and O. A smaller electronegativity difference results in a more covalent bond. 5. A p-orbital is shown below. An electron in this orbital occupies both lobes. How can the electron get from one lobe to the other if the node is a place where the electron can never exist? The electron can behave as a wave, which can have zero amplitude at the node, yet still propagate from one lobe into the other. Node 6. Which of CsF(s) and BaF2 has the higher lattice energy? Give two reasons why. 2 +2 + +2 BaF2 has the higher lattice energy because the Ba has a larger charge than Cs , and because the Ba ion has a smaller radius than the Cs+ ion. Part B. Answer any two of questions B1, B2 B3. If you answer all three, the best two answers will count. (20 marks each) B1. (i) (ii) (iii) (iv) (a) Referring to the structure of ethylene, C2H4, shown below, answer the following questions (10 marks): A is a _sigma_ bond. It is formed by the overlap of an __sp2___ hybrid orbital of one carbon with an _sp2_ hybrid orbital of the other. B is a _pi_ bond. It is formed by the overlap of a _p_ atomic orbital of one carbon with a __p__atomic orbital of the other carbon. C is a _sigma__ bond It is formed by the overlap of an __sp2_ hybrid orbital of the carbon with a ___1s__ atomic orbital of the hydrogen. This molecule has a single / double / triple bond between the carbon atoms (circle one) C B A (b) Use VSEPR theory to predict the shapes of the following four species. Wrong name of shape = zero marks! (5 marks each) (a) KrF4 8 + (4x7) = 36 electrons. Placing Kr at the centre, making four single bonds to the F atoms, and completing the octets around the F atoms uses 32 electrons. There are thus two lone pairs on the Kr atom. The shorthand notation is therefore AX4E2. The shape is therefore square planar. (b) NH4+ 3 5 + (4x1) -1 = 8 electrons. Placing N at the centre and making four single bonds to the H atoms uses all eight electrons. The shorthand notation is therefore AX4. The shape is therefore tetrahedral. B2(i). (10 marks) Photons in the Brackett series of the hydrogen spectrum come from transitions to the m=4 shell from higher shells. Calculate the energy (in kJ/mol) required to remove an electron from the 4th level, i.e. to ionize an atom with an electron initially in the 4th level. Removing the electron requires moving it to infinity. The wavelength of a photon which would be emitted if the electron fell from infinity to the 4th level can be calculated using the Balmer Rydberg equation: 1 1 R 2 2 m n 1 1 1 0.01097 nm 1 2 2 4 0.0006856 nm 1 1 1458 nm 0.0006856 nm 1 And the energy can be found from c 3.00 108 ms 1 6.63 1034 Js 1.36 1019 J(per photon) 1458 109 m 6.02 1023 mol1 82125 J mol1 82.1kJ mol1 Eh B2(ii) (10 marks) (b) The bond energy of F2 molecules is 159 kJ mol-1. Calculate the maximum wavelength of light able to dissociate an F2 molecule. 159 kJ mol-1 = 159,000 J mol-1 159, 000 J mol 1 2.64 1019 J (per photon!) 23 1 6.02 10 mol E h E 2.64 1019 J 3.98 1014 s 1 h 6.63 1034 Js c 3.00 108 m s 1 7.53 107 m 753 nm 14 1 3.98 10 s or, 4 B3. (20 marks) Use the following information to calculate the electron affinity of fluorine (F) in kJ mol-1: 1. 2. 3. 4. 5. The energy change upon combining Li(s) with F2(g) to form LiF(s) is 617 kJ mol-1 The sublimation energy of lithium metal is +121.5 kJ mol-1 The ionization potential of lithium is +520 kJ mol-1 The bond dissociation energy of fluorine gas is +159 kJ mol-1 The lattice energy of LiF(s) is +1050 kJ mol-1 Li(s) Li(g) +121.5 kJ mol-1 Li(g) Li(g)+ + e- +520 kJ mol-1 ½ F2(g) F(g) ½ (+159 kJ mol-1) F(g) + e- F-(g) ?? kJ mol-1 Li+(g) + F-(g) LiF(s) - (1050) kJ mol-1 __________________________________________ Li(s) + ½ F2(g) LiF(s) -617 kJ mol-1 Thus, the electron affinity of F is: ?? = -617 – (121.5 + 520 + ½ (159) – 1050) = -288 kJ mol-1