Lab 7-BAR-Chart-Water
advertisement

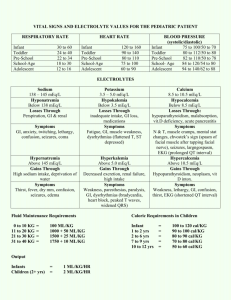
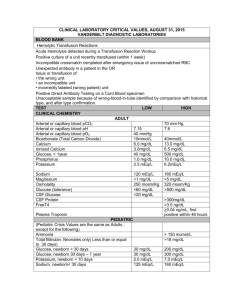
BAR DIAGRAM METHOD FOR WATER SOFTENING PROBLEMS Given a water having the following composition: Ca++ - 40 mg/L = 2 meq/L HCO3¯- 61 mg/L = 1 meq/L Mg++ - 24 mg/L = 2 meq/L SO42¯ - 192 mg/L = 4 meq/L Na+ - 46 mg/l = 2 meq/L Cl¯ - 35.5 mg/L = 1 meq/L These totals must balance + 6 meq/L -6 meq/L a) Put these in the form of a bar diagram expressing all concentrations in terms of CaCO3 (50 mg/L CaCO3 = 1 meq/L) 0 100 200 Ca++ Mg++ HCO3¯ SO42¯ 50 300 Na+ Cl¯ 250 From diagram you can see that Total hardness = 200 mg/L as CaCO3 Ca hardness = 100 mg/L as CaCO3 Mg hardness = 100 mg/L as CaCO3 Total alkalinity = bicarbonate alkalinity = 50 mg/L as CaCO3 So carbonate hardness = 50 mg/L as CaCO3 non-carb. hardness = 150 mg/L as CaCO3 For excess lime softening 1. Add 1 meq/L of Ca(OH)2 to convert HCO3¯ to CO32¯ Ca(OH)2 + Ca (HCO3) 2 → 2CaCO3 ↓ + 2H2O 2. Add 2 meq/L of Ca(OH)2 to provide OH¯ for Mg precipitation Ca(OH)2 + MgSO4 → Mg(OH)2 ↓ + CaSO4 3. Add 1 meq/L excess Ca(OH)2 to raise pH and allow Mg(OH)2 to precipitate. Bar Diagram - 1 Excess 200 150 50 0 Ca Ca Ca OH OH OH 100 Ca 200 Ca Mg HCO30 300 Na SO4 Cl 50 250 → CO32¯ This leaves 200 300 Ca Na OH SO4 Cl 50 250 However in practice not all the CaCO3 and Mg(OH)2 will precipitate. Usually will have 40 mg/L of CaCO3 and 25 mg/L of Mg(OH)2 left so we really have 25 265 Mg Ca OH CO3 75 365 Na SO4 Cl 115 315 For single stage softening we now have a water with a high pH, excess lime and Mg(OH)2. Recarbonation will allow precipitation of CaCO3 and reduce pH. CO2 + Ca(OH)2 → CaCO3 ↓ + H2O CO2 + Mg(OH)2 → MgCO3 ↓ + H2O So add 1 meq/L for excess lime added and 0.5 meq/L for the Mg(OH)2 left. Leaving 25 215 Mg 315 Ca CO3 Na SO4 65 Cl 265 Care must be taken here to avoid reducing pH to the point where CO32¯→ HCO3 as Ca(HCO3)2 will not precipitate. Bar Diagram - 2 This water is still not completely satisfactory as we have (1) CaCO3 still present -- which will precipitate in pipe lines, valves, etc. (2) Non-carbonate hardness (CaSO4) which will prevent soap lathering. For single stage softening we could convert the CO32¯ to HCO3¯ by adding more CO2 at this stage CO2 + CO32¯ + H2O → 2HCO3¯ but this would leave l.3me/l carbonate hardness and 3 me/l non-carbonate hardness. So, go to soda ash for removal of non-carbonate hardness 1. Add 3 meq/L Na2CO3 150 0 190 Na Ca CO3 CO3 290 Na 315 Mg SO4 Cl 65 265 This will leave 40 290 Ca Na CO3 315 Mg SO4 Cl 65 265 To avoid CaCO3 precipitation, now add 1.3 meq/L of CO2 to convert CO3 to HCO3 leaving a water with a composition like this: 40 290 Ca Na HCO3 315 Mg SO4 65 Bar Diagram - 3 Total chemical additions Ca(OH)2 - 4 meq/L = 200 mg/L as CaCO3 Na2CO3 - 3 meq/L = 150 mg/L as CaCO3 CO2 - First stage 1.5 meq/L 2nd stage 1.3 meq/L Total 2.8 meq/L = 140 mg/l as CaCO3 If lime is 95% pure and we treat 10 mgd (Imp) mg 37 1 10 10 = 15,570 #/day Ca(OH)2 l 50 0.95 53 - 150 10 10 = 15,900 #/day pure Na2CO3 50 22 - 140 10 10 = 6,160 #/day CO2 50 Require - 200 This could have been calculated in a manner similar to that in “chemical requirements”. E.g., lime for CO2 - 0 HCO3- - 1 Mg - 2 Excess - 1 4 meq/q Soda ash for non-carbonate hardness - 3 meq/L CO2 - for excess lime - 1 meq/L - for expected residual Mg(OH)2 - 0.5 meq/L - for expected CO32¯ at the end of softening process - l.3 meq/L 2.8 meq/L Note that CO2 additions will depend largely on the final pH desired. They cannot therefore be taken as absolute numbers hut will be adjusted in the treatment plant depending upon the water quality desired. Bar Diagram - 4 In large treatment plants, economies are often affected by removing the sludges and 1. Remixing CaCO3 and Mg(OH)2 sludges in first stage softening and/or the CaCO3 sludges in second stage softening in the flash mixers to aid in flocculation and sedimentation. 2. Heating CaCO3 to produce lime and CO2 (recycling) CaCO3 → CaO + CO2 CaO + H2O → Ca(OH) 2 Water softening sludges may also be used as soil additives in agriculture. Several points must be noted carefully in the use of the above procedures. 1. Equivalent weight of CO2 = 22 (not 44 as you might expect). The equivalent weight of 44 occurs only in reactions such as Na2CO3 + CO2 + H2O → 2NaHCO3 i. e., only a single valence change as CO32-→HCO-. In this case the equivalent weight of Na2CO3 is 106 not 53. 2. Capacity of CO2 equipment will be 7 - 10 times greater than the calculated value as gas transfer efficiency ranges from 10 - 15%. 3. The procedure outlined is for two stages softening. Waters having different quantities of alkalinity and hardness may be treated by: (a) single stage lime softening; (b) excess lime softening; (c) split treatment (only part of the water given excess lime) (d) ion exchange softening. Bar Diagram - 5