Completed Early Career Practitioner Fellowship: Edwin Yan
advertisement

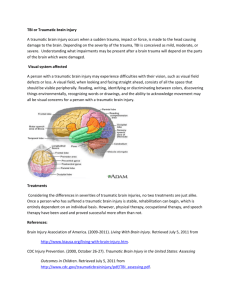
Completed Early Career Research Fellowship: Edwin Yan Host Organisation: Alfred Health TAC Funding: $326,270 Mentors: Professor Jeffrey Rosenfeld, Dr James Bourne & Associate Professor Cristina Morganti-Kossmann Fellowship Project: Establishment a model of traumatic brain injury (TBI) in non-human primate Marmoset and examining the changes in neurotoxin quinolinic acid concentration following TBI Aims: This project investigated whether activation of the kynurenine pathway in the injured brain of both humans and rats contributes to the secondary brain damage that evolves after a TBI. Specifically, this project focused on the production of neurotoxins and potential therapeutical intervention after TBI. 3 studies were undertaken as part of this Fellowship: 1. Investigating neurotoxins in cerebrospinal fluid of TBI patients; 2. in post-mortem human brains after TBI; 3. Validation of the human findings in a rat model. Methods: Study 1: Tryptophan metabolism in human CSF is enhanced by post-traumatic hypoxia. Severe TBI patients with implantation of a cerebro-ventricular catheter for CSF drainage were enrolled in this study. Each day after TBI, CSF was collected into bags kept at 4°C and changed every 24 h until the catheter was removed (n=25). Control CSF samples were obtained from non-TBI, non-cancer, or non-neurodegenerative disease patients undergoing neurosurgery (n=15). The following tryptophan metabolites, tryptophan (TRY), kynurenine (KYN), kynurenic acid (KYNA), 3hydroxykynurenine (3-OHK) and 3-hydroxyanthranilic acid (3-OHAA) were measured by high performance liquid chromatography (HPLC), while quinolinic acid (QUIN), was measured by gas chromatography-mass spectroscopy (GC-MS). Patient’s neurological outcomes were determined by Glasgow Outcome Score Extended (GOSE) and correlated with neurotoxin QUIN concentrations. Study 2: TBI enhances the expression of kynurenine pathway (KP) enzymes in post mortem human brain. Human brain tissues were obtained through the NTFB funded by the VNI. We analysed 21 post-mortem TBI brain samples and 13 brains frominstant non-TBI death. Fresh frozen brain tissues were collected for determining the mRNA levels of KP enzymes (IDO (indoleamine 2,3-dioxygenase), KAT (kynurenine aminotransferase), KYNOH-ase (kynurenine-3-hydroxylase), 3-HAO (3-hydroxyanthranilic acid oxygenase) and QPRT-ase (quinolinic acid phosphoribosy transferase)). Additional brain tissues from the same individuals were fixed in 40% formaldehyde, and then processed for paraffin wax embedding. The tissues were cut into 10 μm sections for immunohistochemistry to identify the localization and distribution of the key enzymes of the KP (IDO and KYNOH-ase). The QUIN containing cells will also be identified by immunohistochemistry. Study 3: Tryptophan metabolism increases in rat brain after TBI, and is exacerbated by post-TBI hypoxia. Diffuse TBI was induced by dropping a 450 grams weight from 2 meters through a vertical tube onto a metal disk fixed onto rat (325-375 grams) skull between bregma and lambda under isoflurane anaesthesia. Four groups of animals were used: (i), 30 min hypoxia only (n=30); (b), TBI only (n=30); (c), TBI followed by a 30 min hypoxia (n=30); and (d), sham operated (n=30). Hypoxia will be induced immediately following TBI by ventilating with a 12% O2 / 88% N2 gas mixture, while for normoxic animals a 22% O2 / 78% N2 gas mixture was used. Animals from eachgroup were euthanized at 2, 12, and 24 hours, 2, and 5 days after treatments (n=6 per time point). Fresh brain tissues were collected from frontal, parietal and occipital cortex for tryptophan metabolite measurements using HPLC and GC-MS. Enzyme expressions of KP (same as in Study 2) were analysed in the same regions described above using real-time-PCR with specific primers for rat. Oxidative stress was determined by measuring malondialdehyde (MDA) levels in brain homogenates. Results: This project has established whether activation of the kynurenine pathway in the injured brain of humans is contributing to secondary brain damage that evolves after TBI. The first study has demonstrated for the first time: (a), the alterations of tryptophan metabolism over 5 days following severe TBI; (b), the effect of post-TBI hypoxia in exacerbating tryptophan metabolism and enhancing neurotoxic QUIN production; and (c), strong correlation between increased QUIN production after TBI and severe neurological outcomes. Through the recently established Neurotrauma Tissue / Fluid Bank (NTFB), we have the unique opportunity to obtain human TBI tissue for in situ detection of molecules of interest. Therefore, Study 2 has provided results for the first time on the level of activation of the kynurenine pathway in human injured brain and the identification of the cellular source of the enzymes involved in this pathway. Study 3 has attempted to use a standardised, reproducible animal model of diffuse TBI in rat. The choice of a diffuse rat brain injury model in combination with post-traumatic hypoxia is clinically relevant since clinical data have shown that majority of severe TBI patients have diffuse type of brain injury. Unfortunately, rats have lacked in QUIN production although the evidences showed the kynurenine pathway was activated following TBI. We have also attempted to measure QUIN concentration in tissues of focal brain injury in mice. The results also showed no changes. These collective evidences have suggested the rodents may not be suitable for investigation of kynurenine pathway after brain injury. Nevertheless, this project has provided further understanding of the kynurenine pathway in the post-TBI human brain, which has demonstrated the potential of blocking neurotoxin QUIN production to reduce secondary brain damage. The better understanding in tryptophan metabolism and enzyme expression following TBI will lead to develop such therapeutical treatment. Conclusions: In this project, we have demonstrated significant increase in QUIN and other intermediate molecule productions. Most importantly, this study has shown for the first time that QUIN increases for at least 15-fold in CSF after TBI. These increases in neurotoxin may contribute to the on-going brain damage after TBI. This study has demonstrated that patients with higher QUIN concentration in the brain soon after TBI had worse neurological outcome at 6 months post-injury. This project had a well understanding on how neurotoxic molecule QUIN been produced after TBI, which may lead to therapeutical protocols aimed at specifically blocking the production of this toxin. Feedback on the Fellowship from Edwin Yan: “I do like to express my sincerely appreciation for TAC and VNI to award this research fellowship. It has tremendously enhanced my research career in traumatic brain injury, advanced my track record and competitiveness in successful in research grants. Through this scheme I have met many internationally recognised researchers in the field of neurotrauma and built numerous collaborations. I also would like to thank all the project managers involved during the period of this fellowship, thank you for your help, patient and encouragement. Finally, I would like to repeat that I had excellent experience and time as a research fellow of TAC” Publications GENG G, JOHNSTON LA, YAN E, BRITTO JM, SMITH DW, WALKER DW, EGAN GF. Biomechanisms for modelling cerebral cortical folding. Med Image Anal. 2009;13:920-930. YAN EB, BABURAMANI AA, WALKER AM, WALKER DW. Changes in cerebral blood flow, cerebral metabolites and breathing movements in the sheep foetus following asphyxia produced by occlusion of the umbilical cord. Am J Physiol Regul Integr Comp Physiol. 2009;297:R60-69. HELLEWELL SC, YAN EB, BYE N, AGYAPOMAA D, MORGANTI-KOSSMANN MC. Posttraumatic hypoxia exacerbates brain tissue damage: Analysis of axonal injury and glial responses. J Neurotrauma 27:1997-2010. MORGANTI-KOSSMANN MC, SEMPLE B, ZIEBELL J, YAN E, BYE N, KOSSMANN T. Modulation of immune response by head injury. In: BERCZI I., ARNASON B, Editors. Neuroimmune Biology: The Brain and host defence. Manitoba, Canada. Elsevier Science; 2010. p.193-210. MORGANTI-KOSSMANN MC, YAN E, BYE N. Animal models of traumatic brain injury: Is there an optimal model to reproduce human brain injury in the laboratory? Injury. 2010;41 Suppl 1:S10-3. YAWNO T, YAN EB, WALKER DW, HIRST JJ. Neuroactive steroids induce changes in foetal sheep behaviour during normoxic and asphyxic insult. Stress. 2011;14( 1):1-5. BYE N, CARRON S, HAN X, AGYAPOMAA D, NG SY, YAN E, ROSENFELD JV, MORGANTIKOSSMANN MC. Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J Neurosci Res 2011:89:986-1000. Presentations ALWIS, COLEMAN, PARKINGTON, ZHANG, MORGANTI-KOSSMANN, YAN. Diffuse traumatic axonal injury induces sensorimotor deficit, memory loss and hippocampal axonal hyperexcitability. Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. KENNA, YAN, GRAY, BOCKING, BRIEN, WALKER, HARDING. Physiologic responses to daily alcohol infusion in the ovine foetus and ewe during late gestation. Society for Gynaecologic Investigation; 2009 March 17-21, Glasgow, Scotland. YAN, GUILLEMIN, WALKER, MORGANTI-KOSSMANN. Increase tryptophan metabolism after traumatic brain injury. International Society for Tryptophan Research; 2009 July 9-11, Florence, Italy. YAN, HELLEWELL, AGYAPOMAA, MORGANTI-KOSSMANN. Post-trauma hypoxia exacerbates neurological deficits, neuroinflammation, and axonal damage in a rat model of diffuse axonal injury. International Neurotrauma Society; 2009 September 7-11, Santa Barbra, USA. BABURAMANI, YAN, WALKER. Prolonged decrease in cerebral blood flow following a transient severe hypoxia in late gestation foetal sheep. Foetal and Neonatal Physiological Society; 2009 September 27-30, Lake Arrowhead, USA. HELLEWELL, YAN, AGYAPOMAA, MORGANTI-KOSSMANN. Post-traumatic hypoxia exacerbates brain damage in an animal model of diffuse axonal injury. Australian Neuroscience Society; 2009 January 27-30, Canberra, Australia. GENG, JOHNSTON, YAN, BRITTO, WALKER, EGAN. Diffusion MR anisotropy predictors of cerebral cortical folding in a foetal sheep model. Australian Neuroscience Society; 1-3 February 2010, Sydney, Australia. ALWIS, COLEMAN, PARKINGTON, ZHANG, MORGANTI-KOSSMANN, YAN. Diffuse traumatic axonal injury induces sensorimotor deficit, memory loss and hippocampal axonal hyperexcitability. Australian Neuroscience Society; 1-3 February 2010, Sydney, Australia. YAN, FRUGIER, LIM, TAN, ROSENFELD, WALKER, GUILLEMIN, MORGANTI-KOSSMANN. Enhanced activation of kynurenine pathway and increased neurotoxin quinolinic acid production following traumatic brain injury in humans. International Neurotrauma Society, 27-30 May 2011, Shanghai, China. HELLEWELL, YAN, BELLANDER, AGYAPOMAA, MORGANTI-KOSSMANN. Post-traumatic hypoxia exacerbates neurological deficit, neuroinflammation and cerebral metabolism in rats with diffuse traumatic brain injury “From Roadside to Recovery – Successful Systems of Trauma Care” Conference, 18-19 November 2011, Melbourne, Australia. YAN, SATGUNASEELAN, HELLEWELL, BYE, ROSENFELD, COOPER, MORGANTIKOSSMANN. A hypoxic insult in patients with traumatic brain injury enhances cerebral inflammatory cytokines, serum biomarkers, and blood brain barrier dysfunction leading to unfavourable outcome. “From Roadside to Recovery – Successful Systems of Trauma Care” Conference, 18-19 November 2011, Melbourne, Australia.