Work With Genetically Modified Animals within BSU`s of Queen Mary
advertisement

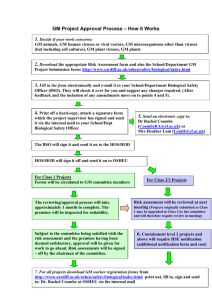
Occupational Health and Safety Directorate Work with Genetically Modified Animals within BSU’s of Queen Mary, University of London THIS DOCUMENT IS INTENDED FOR USE BY ALL PERSONNEL (Staff & Students) WHO INTEND TO WORK WITH GM ANIMALS AND MUST BE COMPLETED AND AUTHORISED PRIOR TO WORK COMMENCING. WHERE NECESSARY (SEE BELOW) IT SHOULD BE ACCOMPANIED BY A GENETIC MODIFICATION RISK ASSESSMENT AS WELL. ENVIRONMENTAL RISK ASSESSMENT AND GUIDANCE Environmental Protection Legislation requires that anyone keeping genetically modified animals (or plants) must carry out an assessment of the risks to the environment. In the case of animals this must include risks arising from the escape of animals. The assessment should also include measures taken by keepers of GMOs to put in place suitable systems, which minimise such damage resulting from any escape. Records of these assessments are to be kept for 10 years. The information in italics below details the parts of the legislation which this document relates to. - “The Genetically modified Organisms (Risk Assessment) (Records and Exemptions) Regulations 1996 (RARER) which implements Section 108 (1) of the Environmental Protection Act, Part VI (EPA), 1990” The College is therefore required by law to ensure that appropriate risk assessments are carried out and appropriate measures are implemented. The Head of Biological Services together with the College GM coordinator are responsible, on behalf of the College, for ensuring suitable measures are in place. All Home Office Project Licence holders must ensure that a risk assessment on the GM animals they wish to use has been carried out. All new Project Licence applications will be required to do this as part of their submission to the appropriate College committee dealing with such applications. Where the generic assessment provided for in this document does not adequately cover the animals to be used and the work to be carried out a further more detailed assessment will be required in order to comply with the Genetically Modified Organisms (Contained Use Regulations) 2000. This generic assessment is intended to include all GM animals (transgenic animals and animals which have been exposed to GM organisms by any means. Where this is unclear individuals investigators must seek advice BEFORE commencing work) that are used within the BSUs of the College unless otherwise stated below. In all cases where animals are exposed to GM organisms, or other vectors, they will have been risk assessed under the GM regulations (at containment level 2) in which case this generic environmental assessment is sufficient. It is expected that the genetic modification in the vast majority of GM animals used within the College will present a negligible risk, particularly when combined with BSU containment measures. GM animals used will be those generated by well established technology designed to produce either a gene knockout or where a gene is over-expressed. Animals will only be obtained from reliable established breeding colonies from authorised suppliers where a detailed history of the animals can be obtained. The primary goal in using GM animals is to understand disease processes and to develop new ways to treat disease. Therefore theses animals will represent a disease model or a modification of such a model which will either increase or decrease disease severity. Where after assessment, the GM animal to be used does not fulfil these criteria and to ensure conformity use of GM animals will not be permitted until the principal investigator concerned has completed a risk assessment for each GM animal. In most cases GM animals will refer to mice, but this can also include rats and other animals in the future. This area will be kept under review and the document modified as appropriate. The key areas to consider are: 1. Physical containment measures to minimise escape. 2. The likelihood that GM animals could cause harm to humans, other animals or plants should they enter the environment. 3. Survivability of the GM animal. 4. Likelihood of crossing with UK species. 5. Ecological niche of the animal. 6. Likely route of transfer to the environment. Risk assessment In all cases where GM animals are to be used within the college BSU facilities an environmental risk assessment must be carried out before work can commence. It is expected that in the vast majority of cases this will be a formality since the risk will be negligible. There is no set proforma, but any risk assessment should consider and include the points above and the following: (1) Aim of the experiment. (2) Source of the genetic material inserted. (3) Details of the GM organism if appropriate. (4) Nature of the gene product, level of expression and site of expression. (5) How the GM animals were generated. (6) Possible impact to the environment. (7) Possible effects on human health. If after this process a particular GM animal is considered to present more that a negligible risk, i.e. the consequences of release into the external environment are more than negligible further measure will be required including a more detailed risk assessment under the aegis of the GM regulations. Investigators should refer to the College Health and Safety website for further information and contacts names. Physical containment measures to minimise escape All BSUs within the College where GM animals (as defined above) are used provide a high level of containment to reduce the risk of animals escaping into the external QM/H&S/0092 07/01/ 2008 (V 1.1), Approved by GMSC 19/05/2008 environment to a negligible level. (a) All rooms where GM animals are housed are fitted with rodent barriers. (b) All external access doors to the Units are fitted with rodent barriers. (c) Drains which can potentially provide an escape route to animals are protected by wire meshing. (e) Infrastructural changes which may take place within Units must take containment, measures into account as a priority. (f) All reasonable security measures must be taken to ensure that malicious acts do not result in the deliberate release of GM animals. Access to BSUs and rooms therein should only be for authorised personnel. (g) Cages must be maintained in a good state of repair. (h) Room fabric must be kept clean and free of unnecessary rubbish as well as being in a good state of repair. The likelihood that GM animals could cause harm to humans, other animals or plants should they enter the environment In the extremely unlikely event that an animal does escape into the external environment it is considered to be a negligible risk, and would not therefore pose any more risk than existing wild animals. Where a potential risk has been identified the case will be referral to the GM local committee for further information and assessment. Survivability of the GM animal It is expected that the survivability of GM animals in the external environment will be low, since in the majority of cases animals used will be specifically bred laboratory strains and housed in ideal living conditions (heat light food bedding etc) These animals will also be more susceptible to predation and disease. Where a potential risk has been identified the case will be referred to the GM local committee for further information and assessment. Likelihood of crossing with UK species It is expected that the likelihood of crossing with UK species will be low, since in the majority of cases animals used will be specifically bred laboratory strains which often have low fecundity. However it is accepted that a small risk here does exist. Where a potential risk has been identified the case will be referred to the GM local committee for further information and assessment. Ecological niche of the animal Although in principle GM animals would find a suitable niche within the external environment it is considered extremely unlikely that this would present an environmental hazard since they would present no more additional problems than might be encountered by non-manipulated and indigenous species. Likely route of transfer to the environment Although it is possible that genetic material could be transferred between compatible species the likelihood of adverse environmental consequences is considered extremely low. In the majority of cases the nature of genetic modification will be considered to be harmless and in some cases deleterious to the host animal, thus hastening its eradication from the environment. Where a potential risk has been identified the case will be referred to the GM local committee for further information and assessment. QM/H&S/0092 07/01/ 2008 (V 1.1), Approved by GMSC 19/05/2008 This document is intended to provide guidance and a generic environmental risk assessment which is designed to cover the majority of GM animals used within the College. Since a high level of containment is available with the College BSUs it is expected that in only a small minority of cases, where work with GM animals is being carried out, will a further review of the assessment be required. Compliance with other legislation It is important that project licence holders carrying out work as described above ensure that all personal licence holders understand and are fully aware of the contents of this document. Additionally is expected that ALL investigators working with GM animals will have complied with GM, COSHH, Home Office and other appropriate regulations. Declaration I have read, understood and agree to comply fully with the above. The GM animals used under my Home Office Licence comply with this generic assessment. NAME …………………………………….. DEPARTMENT/DIVISION …………………………………….. HOME OFFICE LICENCE PROJECT NUMBER SIGNED ………………………………… DATE …………………………….. …………….. ------------------------------------------------------------------------------------------------------GM COORDINATOR SIGNED …………………………………….. ………………………………… DATE …………….. HEAD OF BIOLOGICAL SERVICES …………………………………….. SIGNED ………………………………... DATE …………….. Copies of this signed document will be kept by the College GM co-ordinator, the Chair of the local GM safety committee and the Head of Biological services. FOR GUIDANCE, AND WHERE TO OBTAIN FURTHER HELP Investigators should refer to the College Health and Safety website for further information and contacts names. For the full (official) text of the Genetically modified Organisms (contained Use) Regulations 2000, plus detailed explanations and guidance, you may find it useful to refer to the booklet "A Guide to the Genetically Modified Organisms (Contained Use) Regulations 2000" (HSE Books, ISBN 0-7176-1758-0) copies of which are held by the College GMSC Chair, GMSC Coordinator and the College Safety Advisers. You should also consult the SACGM Compendium (http://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/index.htm QM/H&S/0092 07/01/ 2008 (V 1.1), Approved by GMSC 19/05/2008 of ). Guidance The Compendium gives advice on how to undertake the required risk assessment. It also gives guidance on the containment measures required and the classification of activities with GM animals (Part 5). Further information can also be found at the HSE website (www.HSE.gov.uk ). The SACGM newsletters can also be found at http://www.hse.gov.uk/aboutus/meetings/sacgmcu/meetings.htm Details of the Environmental Protection Act 1990 (c43) can be found at http://www.hmso.gov.uk/acts/acts1990/Ukpga_19900043_en_1.htm (copies can be obtained from HM Stationers ISBN 0105443905). For further guidance on the categorisation of biological agents please see: Advisory Committee on Dangerous Pathogens (ACDP) http://www.hse.gov.uk/pubns/misc208.pdf QM/H&S/0092 07/01/ 2008 (V 1.1), Approved by GMSC 19/05/2008