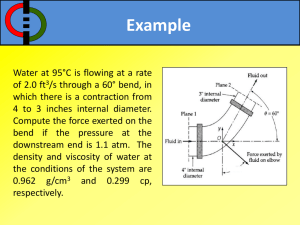
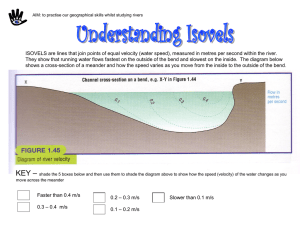
Materials and Methods. (doc 39K)
advertisement

Supplementary materials and methods Flow cytometry bEnd.3 cells were cultured on 60 mm tissue culture dishs in 10% FBS, high glucose DMEM and treated with 20 ng/ml recombinant mouse TNFα (Peprotech, Inc., Rocky Hill, NJ) for 4 and 16 h. The TNFα-treated bEnd.3 cells were detached from cell culture dish with cell dissociation buffer (Versene, Invitrogen), incubated with 1% BSA in DMEM for 30 min at room temperature, followed by 2 µg/ml goat anti-VCAM-1 or MAdCAM antibody (R&D systems, Inc., Minneapolis, MN) on ice, and washed in DMEM twice. Cells were then incubated in 2 µg/ml of Alexa 488 donkey anti-goat IgG antibody. 10,000 events were analyzed by flow cytometry (Coulter EPICS XL-MCL) with a 488 nm argon ion laser. Cell coating and binding assay on mouse brain endothelial cells (bEnd.3) Palmitated protein G (PPG) was derivitized as described previously by reacting recombinant protein G (Pierce, Rockford, IL) and N-hydroxysuccinimide ester of palmitic acid (Sigma, St. Louis, MO) followed by purification using Sephadex G-25 (Pharmacia, Piscataway, NJ). Cell coating and cell binding assay was conducted as previously described [6]. Mouse brain endothelia cells (bEnd.3; ATCC) were cultured at 37⁰C, 5% CO2 in high glucose DMEM supplemented with 10% FBS, penicillin and streptomycin. For cell attachment studies, 80 µl of 5 X 105 cells/ml of bEnd.3 were seeded onto thermanox® plastic coverslips (13 mm round slips, Ted Pella, Inc, Rochester, NY), transferred into a 24-well microplate, and cultured for 1 day prior to adherence assays. bEnd.3-seeded coverslips were blocked with 1% BSA in DMEM in a 37⁰C, 5% CO2 incubator for 30 min, and then incubated for 30 min with 80 µl of 5 X 105 cells/ml, followed by washing with DMEM three times and counted with fluorescent microscope. Incorporated Antibody release test from cell membrane Cells were coated with PPG and human FITC-conjugated IgG according to standard methods, and the fluorescent intensity measured using flow cytometry. To avoid loss of signal due to cell spreading and the need to trypsinize, Immediately after coating, aliquots of 1.0 x 10^6 cells were placed in 1 ml aliquots of complete medium (10% FBS in DMEM) containing 2% alginate. The cell/alginate mixture was then injected into complete medium containing 50 mM calcium chloride and allowed to gel for 10 minutes at room temperature, the medium was then exchanged to complete medium and the cells incubated at 37 degrees. For flow cytometric analysis, the cells were incubated in complete medium containing 2 mM EGTA to release the cells from the alginate. Supplementary data Figure S1. Preparation of Fluc-MSC. Murine MSCs (BMC9 cells) were transduced with a triplefusion reporter, fluc-mrfp-ttk (encoding firefly luciferase, monomeric red fluorescent protein (RFP), and truncated herpes simplex virus type 1 sr39 thymidine kinase) by use of a lentiviral vector showed, and showed 96.7% positive for RFP expression by flow cytometry at the University of Pittsburgh) (Fig. S1-a). Fluc-MSCs plated onto 96 well plates and assayed for luciferase activity (Fig. S1-b) showed a linear correlation between cell numbers and luciferase activity assay (Fig. S1-c). Figure S2. MSC localization in lung and colon 2 hours post-injection. MSCs labeled with FarRed dye were injected into IBD treated mice and the mice harvested after 2 hr (not sure of this time), tissues frozen, sectioned and immunostained. Arrows point to MSCs (red) and green indicates immunostaining with for either laminin (A, B) to highlight basement membrane from colon or von Willibrand factor (C, D) to label endothelial cells. The MSC in A looks to be inside of a small capillary, the MSC in D appear to be lining a large blood vessel or air space, while the MSCs observed in B and C are localized to regions within the mesenchyme. C = capillary; BV = blood vessel. Figure S3. Quantification of antibody incorporation on MSCs. An FITC-labeled human IgG standard curve was produced (a), and the fluorescent intensity of FITC-antibody coated-MSCs was graphed vs cell number (b) and the amount of incorporated antibody on MSCs was calculated by comparing fluorescent intensity from antibody-coated MSCs with that of standard curve (c). Figure S4. Schematic diagram of effective targeting Ab-MSCs to inflammatory sites. The enhanced therapeutic effects are mainly mediated by increased MSC delivery to sites of inflammation and also derived from blocking T cells adhesion to endothelium by locally released antibody from MSC membrane. Figure S5. MSC binding to bEnd.3. Cell binding assay of MSCs on bEnd.3 cells showed a 8fold increase in cell binding of AbVCAM-1-MSCs compared to MSCs only (* p<0.05) and PPGMSCs (** p<0.05) and a 3-fold compared to untreated and TNFα treated bEnd.3 cells († p<0.05). Figure S6. Addressin expression on bEnd.3 cells. Flow cytometric results showing VCAM-1 (a) and MAdCAM (b) expression in bEnd.3 cells, a mouse endothelial cell line, after 20 ng/ml of TNFα treatment for 4 h or 16 h; VCAM-1 expression was upregulated while MAdCAM expression was unchanged. (c) Quantification of addressin expression based on fluorescent intensity from flow cytometric results. bEnd.3 cells showed 4-times higher expression than untreated cells by TNFα treatment(* p<0.05): while MAdCAM expression was unchanged; (N=3, Student’s t-test). Figure S7. Antibody release test from cell membrane. Cells were coated with FITC-labeled human IgG and after each incubation time at 37⁰C, FITC fluorescent intensity on cells was analyzed by flow cytometry. The results show a FITC-IgG half-life of 3.4 hr; error bars show standard deviation; N = 4.