Polylactic acid (PLA) is a biocompatible and biodegradable polymer
advertisement
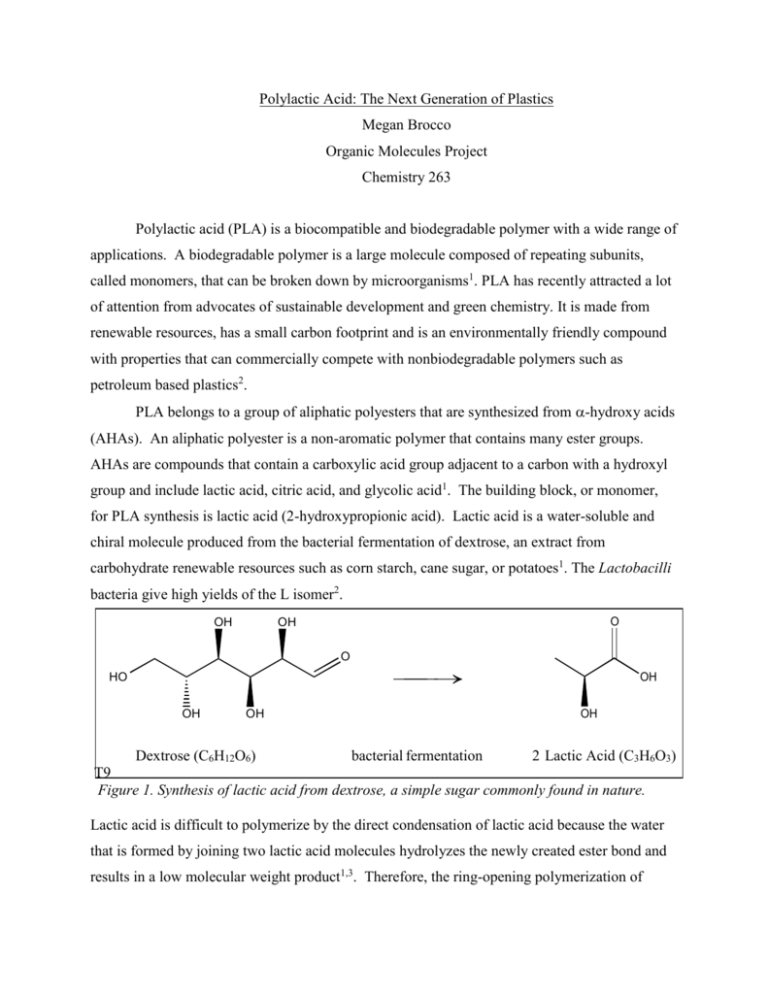
Polylactic Acid: The Next Generation of Plastics Megan Brocco Organic Molecules Project Chemistry 263 Polylactic acid (PLA) is a biocompatible and biodegradable polymer with a wide range of applications. A biodegradable polymer is a large molecule composed of repeating subunits, called monomers, that can be broken down by microorganisms1. PLA has recently attracted a lot of attention from advocates of sustainable development and green chemistry. It is made from renewable resources, has a small carbon footprint and is an environmentally friendly compound with properties that can commercially compete with nonbiodegradable polymers such as petroleum based plastics2. PLA belongs to a group of aliphatic polyesters that are synthesized from -hydroxy acids (AHAs). An aliphatic polyester is a non-aromatic polymer that contains many ester groups. AHAs are compounds that contain a carboxylic acid group adjacent to a carbon with a hydroxyl group and include lactic acid, citric acid, and glycolic acid1. The building block, or monomer, for PLA synthesis is lactic acid (2-hydroxypropionic acid). Lactic acid is a water-soluble and chiral molecule produced from the bacterial fermentation of dextrose, an extract from carbohydrate renewable resources such as corn starch, cane sugar, or potatoes1. The Lactobacilli bacteria give high yields of the L isomer2. OH O OH O HO OH OH OH Dextrose (C6H12O6) OH bacterial fermentation 2 Lactic Acid (C3H6O3) T9 Figure 1. Synthesis of lactic acid from dextrose, a simple sugar commonly found in nature. Lactic acid is difficult to polymerize by the direct condensation of lactic acid because the water that is formed by joining two lactic acid molecules hydrolyzes the newly created ester bond and results in a low molecular weight product1,3. Therefore, the ring-opening polymerization of lactide, a cyclic di-ester, is the most common method used to synthesize PLA3. In this esterifcation reaction, lactic acid is heated with an acid catalyst to form a dimer which then converts to lactide, a hexagonal ring with two ester groups1,4. O 1) Condensation H20 OH 2) Acid Cat. ∆ OH Figure 2. Synthesis of lactide, a cyclic diester, from lactic acid. O O O O O O L-Lactide O O O O O O Meso-Lactide R-Lactide Figure 3. Lactide synthesis results in three stereoisomers because lactic acid has a chiral center. O O Nuc O O O O Nuc O O O O O O O O O O O O O O O O O O O O O Figure 4. Ring opening polymerization of lactide showing nucleophilic and electrophilic sites. O Typically tin octoate or stannous octoate are used as catalysts for this reaction3. This method of synthesis greatly reduces the use of expensive and harmful solvents and results in a product that is biodegradable. In the presence of microorganisms and oxygen, PLA will naturally degrade to non-toxic carbon dioxide and water within a few weeks1. In addition, the ester linkages in PLA can be easily hydrolyzed with water to form lactic acid, the monomer for PLA synthesis. The hydrolysis is typically catalyzed by the carboxylic acid group located at the end of the chain that has a pKa of 33. This way, the PLA monomer can be recycled and used to make more PLA3. Figure 5. Life Cycle of polylactic acid. (http://www.toyo-eng.co.jp/en/product_line/environment/baiomass/index.html) The physical properties of PLA vary because the building blocks used in its synthesis come in different forms. For example, lactic acid has two optical isomers, L-lactic acid and Dlactic acid, while lactide has three optical isomers, L-lactide, D-lactide, and meso-lactide. This allows the properties of PLA to be modified by changing the ratio of L and D enantiomers of lactic acid used in the synthesis which affects the degree of crystallinity and many other important properties such as density, molecular weight, melt temperature, strength, and glass transition1, 3,5. Depending on the L/D ratio, PLA can be semi-crystalline or amorphous, which reflects the structure of the polymer’s carbon backbone. In general, PLA behaves like polyethylene terephtalate (PET or PETE), a recyclable amorphous to semi-crystalline thermoplastic polymer that we commonly find in synthetic fibers, containers, and plastic bottles4. Like PLA, PET is synthesized from an esterification reaction. However, it’s monomer, bis-Bhydroxyterephthalate, is made using p-xylene, acetic acid solvent, cobalt and manganese catalysts, and bromide or ethylene glycol, dimethyl terephtalate and methanol instead of renewable resources6. PLA also acts like polypropylene, a tough and flexible thermoplastic polymer found in packaging, industrial texiles, piping, heat-resistant plastics, and floating rope7. In addition, the properties of PLA can be customized by adding plasticizers, biopolymers, and other fillers2. The variety of structures and physical properties that result from modifying all of these variables has therefore been the subject of much research in the scientific community. Chemists have been working hard to tailor the properties of PLA to make it practical for a wide variety of applications in addition to exploring the endless list of PLA blends that can be created using other polymers. A diverse assortment of products can be manufactured from PLA because its properties allow it to be stress crystallized, thermally crystallized, filled, and processed in many polymer manufacturing plants3. Biodegradability and non-hazardous materials makes PLA a great choice for post-consumer products that typically end up in landfills like plastic bags, food containers, and other packaging. Products can be fabricated from PLA by the following processes: injection molding (plastic bottles), sheet extrusion (plastic containers and truck bed liners), blow molding (hoses and pipes), film forming (wrappers) and fiber spinning (carpeting and fiberfill)4. In addition, the biocompatible properties of PLA allows it to be in contact with tissues and have many applications in the biomedical field8,9 Currently, PLA is used in the manufacture of sutures, stents, dialysis equipment and other medical devices. PLA has also found uses in tissue engineering where it is used as the artificial scaffold to support the three-dimensional growth of cells ex vivo and in vivo.10 The scaffold gives cells a place to attach and grow, allows diffusion of nutrients, and gives support to the structure and shape of the growing tissue11. This application requires strong but malleable porous materials that are biocompatible and biodegradable so that the scaffold does not need to be surgically removed after the tissue has formed. PLA is even more competitive because it biodegrades into lactic acid, a naturally occurring compound that is easily removed from the body. In conclusion, PLA is a promising polymer with a wide variety of physical properties and applications. PLA is currently used in the production of packaging, textiles, and biomedical devices. PLA is similar to other petroleum-based polymers on the market but has a competitive edge because of the growing demand for non-toxic, environmentally friendly, and sustainable products that do not increase emissions or fill up landfills. PLA differs from these other recyclable plastics because it is made from renewable resources and biodegrades into naturally occurring compounds12. The biomedical and tissue engineering fields are also utilizing PLA’s unique properties especially because it is biocompatible and degrades. The production cost of PLA has been falling, allowing it to become more competitive with other polymers on the market. Cost no longer seems to be a factor limiting the use of PLA. Many scientists are focusing research on PLA and exploring the properties that can be achieved in the lab. Overall, it looks like PLA has the potential to change to plastics industry and that we will see a lot more of PLA in the future. References 1 Bruice, P.Y. (2011). Organic Chemistry. Glenview, IL: Prentice Hall. 2 Averous, L. (2008). Polylactic Acid: Synthesis. Properties, and Applications. Retrieved from http://www.biodeg.net/fichiers/Polylactic%20Acid%20Synthesis%20Properties%20and% 20Applications.pdf 3 Henton, D., et al. Polylactic Acid Technology. (2005). Retrieved from http://www.jimluntllc.com/pdfs/polylactic_acid_technology.pdf 4 Wikipedia. (2011). Polylactic acid. Retrieved from http://en.wikipedia.org/wiki/Polylactic_acid 5 Södergård, A., & Stolt, M. (2002). Properties of lactic acid based polymers and their correlation with composition. Progress in Polymer Science, 27 (6): 1123–1163. doi:10.1016/S00796700(02)00012-6 6 Wikipedia. (2011). Polyethylene terephthalate Retrieved from http://en.wikipedia.org/wiki/Polyethylene_terephthalate 7 Wikipedia. (2011). Polyproylene. Retrieved from http://en.wikipedia.org/wiki/Polypropylene 8 Middelton, J., & Tipton, A. (2000). Synthetic biodegradable polymers as orthopedic devices. Biomaterial, 21 (23), 2335–2346. doi:10.1016/S0142-9612(00)00101-0 9 Moran, J., et al. (2003). Characterization of Polylactic Acid–Polyglycolic Acid Composites for Cartilage Tissue Engineering Tissue Engineering, 9 (1). Retrieved from http://www.mae.cornell.edu/PDF/lb244/moran2003.pdf 10 Chen, G., et al. (2002). Scaffold Design for Tissue Engineering. Macromolecular Bioscience, 2, 67-77. Retrieved from http://web.mit.edu/course/3/3.042/team1_06/reference/Chen%20%20scaffold%20design%20for%20tissue%20engineering.pdf 11 Tu, C., et al. (2003). The fabrication and characterization of poly(lactic acid) scaffolds for tissue engineering by improved solid–liquid phase separation. Center for Molecular Sciences, Institute of Chemistry, Chinese Academy of Science. Retrieved from http://www.uta.edu/faculty/jianyang/publications%20PDF/PAT%20TU%202003.pdf 12 Royte, E. (2006, August). Corn plastic to the rescue. Smithsonian. Retrieved from http://www.smithsonianmag.com/science-nature/plastic.html




