UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY
advertisement

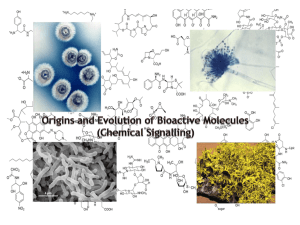
UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE MODULE SPECIFICATION TEMPLATE 1 The title of the module BI614 Topics in Medical Biosciences 2 The Department which will be responsible for management of the module Biosciences 3 The Start Date of the Module January 2007 4 The number of students expected to take the module 60 5 Modules to be withdrawn on the introduction of this proposed module and consultation with other relevant Departments and Faculties regarding the withdrawal None this is a revising of an existing module 6 The level of the module (eg Certificate [C], Intermediate [I], Honours [H] or Postgraduate [M]) H 7 The number of credits which the module represents 15 8 Which term(s) the module is to be taught in (or other teaching pattern) Term 2 9 Prerequisite and co-requisite modules Prerequisite for Haematology and Cellular Pathology are BI627 Haematology and Blood Transfusion and BI525 Introduction to Laboratory Medicine A Prerequisite for Biochemical Investigation of Human Disease is BI626 Integrated Endocrinology and Metabolism 10 The programmes of study to which the module contributes Biomedical Science Biomedical Science with a Sandwich Year Biochemistry with Medical Biosciences 11 The intended subject specific learning outcomes and, as appropriate, their relationship to programme learning outcomes 116096067 UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE This module comprises several components covering key areas relevant to Medical Biosciences. Topics are delivered by staff working in the chosen areas and reflect current research and/or applications in Medical Biosciences. On successful completion of this module students will have achieved three of the following: - Haematology and Cellular Pathology *- an understanding of how certain disease states affect haematological values and a knowledge of the treatment regimes used to treat them; an understanding of the specialised techniques used in Cellular Pathology (Programme outcomes 10,13) - Cancer: Biology and Treatment - An appreciation that cancer is a collection of diseases with different clinical manifestations, risks and treatments; an understanding of how molecular changes are responsible for the development of individual cancers and associated with time to relapse and death; a knowledge of the ethic issues that arise from out greater understanding of the molecular biology of the disease. (Programme outcomes 5,10,16,24) - Molecular Medicine - the ability to discuss the advantages and disadvantages of both viral and non-viral methods for the delivery of nucleic acids for gene therapy; the ability to describe the role played by inappropriate cell death or survival in selected disease states and how an understanding of these processes has led to the development of experimental strategies for the treatment of human disease; the ability to explain the roles played by proteomics, functional genomics and nucleic acid based diagnostics in the discovery of novel therapeutic targets and agents. (Programme outcomes 4,5,24) - Bioactive Molecules and Biomedicine -An appreciation that a range of molecules produced by microorganisms have bioactive properties; a knowledge of how selected bioactive molecules exert their effect on metabolic processes; the ability to identify useful synthetic activities present in microorganisms and to use existing information in design search strategies to find new bioactive molecules produced by microorganisms (Programme outcomes 8,9) - Epidemiology and Disease* - A knowledge of microorganisms of medical importance, how the spread of disease is monitored in animal and human populations and the principles of prevention and containment for various 116096067 UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE diseases; an understanding of the pathogenic mechanisms of specific organisms and the importance of vaccination and immunisation policies at local and national levels. (Programme outcomes 7,8,15) - Biochemical Investigation of Human Disease* - A knowledge of the principles underpinning biochemical screening; an ability to discuss the application of biochemical techniques to the detection and management of human disease and the impact of genetic and environmental factors on the development of human disease.(Programme outcomes 9,11) Topics marked * are compulsory for students taking the accredited Biomedical Science degree programme 12 The intended generic learning outcomes and, as appropriate, their relationship to programme learning outcomes The following generic learning outcomes are achieved in all of the options available 13 - the ability to retrieve, analyse and evaluate information from text books, - the development of written communication skills (33) primary research papers and review articles (19,28) A synopsis of the curriculum - Haematology and Cellular Pathology – antiphospholipid syndrome , advances in anticoagulation; Von Willibrands disease and its treatment, factor XII – essential or irrelevant, use of gene therapy in the treatment of Haemophilia and the development and use of “artificial blood” - Procedures for specific substances and tissues, methods for tissue markers and products, cytopreparatory techniques, diagnostic cytology, fine needle biopsy, post mortem techniques and tissue display - Cancer : Biology and Treatment – incidence, causes and prevention of cancer; the pathology of cancer; growth regulation and intracellular signalling; oncogenes and mechanisms of cell transformation; tumour suppressor genes and genetic instability; metastasis; inherited predisposition to cancer; current and new treatments for cancer; ethical issues in cancer. - 116096067 Molecular Medicine – physical methods for gene delivery; viral vectors for gene therapy; antisense technology and ribozymes; functional genomics and UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE proteomics; apoptosis in disease; anti-CD33 engineered lymphocytes for the treatment of myeloid leukaemia. - Bioactive Molecules and Biomedicine - types of bioactive molecules; secondary metabolites and antibiotics – their synthesis by microbes; mechanisms of antibiotic action; other pharmacologically active microbial products; β lactam antibiotics; the use of genetically modified bacteria; biotransformations and strategies for finding new bioactive agents. - Epidemiology and Disease – the epidemiology and prevention of Salmonella, Diphtheria, Influenza, Zoonotic diseases, Meningitis and Dermatophyte infection; the epidemiology of Staphylococcus – old pathogen or emerging problem?; methodology used in epidemiology. - Biochemical Investigation of Human Disease – screening for disease – concepts, rationale and screening programmes, application of biochemical techniques to paediatrics and inborn errors of metabolism, tumour markers, liver function, iron and porphyries, enzymes and their use in laboratory medicine, clinical applications of protein biochemistry, nutrition in health and disease, lipids and atherosclerosis. 14 Indicative Reading List - Haematology and Cellular Pathology Essential Haematology – A.V. Hoffbrand, S.M. Lewis and E.G.D. Tuddenham Haemostasis and Thrombosis – T.G. Deloughery Relevant research papers will be recommended during the course - Cancer: Biology and Treatment Cancer Biology (2nd Edition) R.J.B. King - Molecular Medicine Lecture Notes on Molecular Medicine (2001) J. Bradley et al Human Molecular Genetics 2 (1999) T. Strachan and A.P. Read Essentials of Medical Genomics (2003) S.M. Brown - Bioactive Molecules and Biomedicine Discovery and Isolation of Microbial products (1985) M.S. Verrall The Biosynthesis of Secondary Metabolites (1989) R.B. Herbert Biotransformations (2000) K. Faber 116096067 UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE - Epidemiology and Disease Medical Microbiology (1998) Mims, Playfair, Roitt, Wakelin and Williams Principles of Bacteriology and Immunity, Topley and Wilson - Biochemical Investigation of Human Disease Clinical Biochemistry (3rd Edition) A. Gaw, M.J. Murphy, R.A. Cowan, D.S.J. O’Reilly, M.J. Stewart and J. Shepherd. Clinical Biochemistry Metabolic and Clinical Aspects. W.J. Marshall and S.K. Bangert (Eds) 15 Learning and Teaching Methods, including the nature and number of contact hours and the total study hours which will be expected of students, and how these relate to achievement of the intended learning outcomes Contact Hours – 28 hours (lectures ) Self Study - 122 hours ( recommended reading 30 hours, preparation for assessment 30 hours and preparation for examination 62 hours) 16 Assessment methods and how these relate to testing achievement of the intended learning outcomes Coursework 30% - Each of the options has a written assessment to test achievement of the subject specific and generic learning outcomes for each option. Students complete a total of three assessments according to their chosen options, each assessment is worth 10% of the module mark. - Haematology and Cellular Pathology - A short essay - Cancer: Biology and Treatment A short essay on a current issue in cancer ( 400 word summary) - Molecular Medicine - Journal club – preparation of a presentation based on an allocated research - Bioactive Molecules and Biomedicine - - - Epidemiology and Disease - paper Report on a selected class of bioactive molecules essay based on the problems relating to an infectious agent and the mechanism to 116096067 control /eradicate the disease from the patient. UNIVERSITY OF KENT – CODE OF PRACTICE FOR QUALITY ASSURANCE - Biochemical Investigation of Human Disease Structured review of a published article Examination 70% 17 Implications for learning resources, including staff, library, IT and space No additional resources are required 18 A statement confirming that, as far as can be reasonably anticipated, the curriculum, learning and teaching methods and forms of assessment do not present any non-justifiable disadvantage to students with disabilities As far as can be reasonable anticipated, the curriculum, learning and teaching methods and forms of assessment do not present any non-justifiable disadvantages to students with disabilities. Statement by the Director of Learning and Teaching: "I confirm I have been consulted on the above module proposal and have given advice on the correct procedures and required content of module proposals" ................................................................ Director of Learning and Teaching .............................................. Date Statement by the Head of Department: "I confirm that the Department has approved the introduction of the module and will be responsible for its resourcing" ................................................................. Head of Department 116096067 .............................................. Date