quiz3-0506ans
advertisement

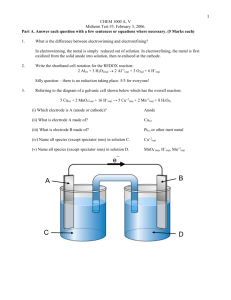
1 CHEM 1000 A, V Midterm Test #3, February 3, 2006 Calculator allowed. This test should have seven pages. A periodic table, some constants and a table of reduction potentials are on the last page. Part A. Answer each question with a few sentences or equations where necessary. (5 Marks each) 1. What is the difference between electrowinning and electrorefining? Electrowinnig refers to the reduction of metals from solution to the solid state. Electrorefining starts with impure metal, oxidizes it into solution, then reduces it back to the pure solid state. 2. Write the shorthand cell notation for the REDOX reaction: Cr2O7-2(aq) + 6 Br(aq) + 14 H+(aq) 2 Cr+3(aq) + 3 Br2(l) + 7 H2O(l) Pt(s) | Br-(aq) | Br2(l) || Cr2O7-2(aq), Cr+3(aq), H+(aq) | Pt(s) 3. Referring to the diagram of a galvanic cell shown below which has the overall reaction: 5 Al(s) + 3 MnO4-(aq) + 24 H+(aq) → 5 Al+3(aq) + 3 Mn+2(aq) + 12 H2O(l) (i) Which electrode is A (anode or cathode)? __Anode_________________________ (ii) What is electrode A made of? __Al(s)_________________________ (iii) What is electrode B made of? __Pt(s), likely_____________________ (iv) Name all species (except spectator ions) in solution C. __Al+3(aq)_________________________ (v) Name all species (except spectator ions) in solution D. __MnO4-(aq), Mn+2(aq), H+(aq)_____________ e¯ A B C D 2 4. In an electrolytic cell having two Platinum electrodes and NiF2(aq) as an electrolyte, what is produced at the anode and what is produced at the cathode? At the anode, the two possible reactions are: 2 F-(aq) F2(g) + 2 e- Eo = -2.87 V 2 H2O(l) O2(g) + 4 H+(aq) + 4 e- Eo = -1.23 V Since the second reaction has the more positive potential, O2(g) and H+(aq) are produced at the anode. At the cathode, the two possible reactions are: Ni+2(aq) + 2 e- Ni(s) Eo = -0.26 V 2 H2O(l) +2 e- H2(g) + 2 OH-(aq) Eo = -0.83 V Since the first reaction has the more positive potential, Ni(s) is produced at the cathode. 5. Name the strongest intermolecular force between two molecules of CS2. Dispersion forces. 6. Referring to the phase diagram for carbon below, does the melting point of graphite increase or decrease as the pressure is increased? How do you know? The S-L equilibrium line between graphite and liquid carbon has a positive slope, therefore the melting point of graphite increases as the pressure is increased. 106 Diamond Liquid 104 p, atm 102 Graphite Gas 1 0 6000 T, K Part B. Answer any three questions. If you answer all four, the best three will be used to calculate your mark. (20 marks each) B1. In a fuel cell which consumes methanol and oxygen, the reactions are: CH3OH(aq) + H2O(l) → CO2(g) + 6H+(aq) + 6eand + 1.5 O2(g) + 6H (aq) + 6e- → 3H2O(l) (a) Write the overall reaction. CH3OH(l) + 1.5 O2(g) CO2(g) + 2 H2O(l) 3 -1 (b) Calculate the standard free energy change of the overall reaction (kJ mol ), assuming the standard cell potential is +1.210 V. Go = -nFEo = - 6 (96487 C/mol)(1.210 J/C) = -700496 J/mol = -700.5 kJ/mol (c) Calculate the potential of this fuel cell (in Volts) at -50oC if [CH3OH(aq)] = 10.0 mol L-1, pO2( g ) = 0.2 atm and pCO2( g ) = 0.001 atm. E Eo Q RT ln(Q) nF p CO2 [CH 3OH (aq) ]p1.5 O2 0.001 10(0.2)1.5 0.00112 Thus, E 1.210 V 8.314 J K 1mol1 (273.15 50)K ln(0.00112) 6(96487 C mol1 ) 1.232 V B2. Copper metal is being electroplated from a solution of CuCl2(aq). The cathode is a thin steel rectangle 1 m wide and 2 m high. How thick (in cm) will the copper metal be if the solution is electrolyzed using a current of 1000 A for 48 hours? Note that the metal is being plated onto both sides of the cathode simultaneously. The density of copper metal is 8.92 g cm-3. it = nF it F 1000 C s 1 (48 h 3600s h 1 ) 96487 C mol 1 n 1790.9 mol (of electrons) But the reduction reaction is Cu+2(aq) + 2 e- Cu(s), so 1 mole of electrons produces only 0.5 mol Cu(s). Thus, moles Cu(s) produced = 1790.9 / 2 = 895.5 mol Cu(s). 65.5 g/mol = 56862 g Cu(s) 8.92 g cm-3 = 6325 cm3 Cu(s) Area of cathode = 100 cm x 200 cm x 2 (since there are two sides) = 40000 cm2. 4 3 2 Thickness of Cu(s) = 6325 cm / 40000 cm = 0.159 cm. B3. (a) Predict which of 2-propanol or 2-proanone has the higher boiling point and explain why. O OH C H3C C CH3 H 2-propanol H3C CH3 2-propanone We expect 2-propanol to have the higher boiling point since it experiences H-bonds, whereas the 2propanone experiences relatively weaker dipole forces only. (b) Now calculate the boiling points of 2-propanol and 2-propanone (in oC) from the following data: Enthalpy of Vaporization Hvap, kJ mol Vapor Pressure at 25oC, mm Hg -1 2-propanol 42.1 42.8 2-propanone 31.9 221.0 Rearranging the Clausius-Clapeyron equation gives: 1 p R T2 ln 2 T1 H vap p1 1 Here, T1 is 298.15 K (25oC), and p1 is the vapor pressure at 25oC T2 is the boiling point, and p2 = 760 mm Hg, since the normal boiling point is defined at 1 atm pressure. For 2-propanol, 1 p R T2 ln 2 T1 H vap p1 1 1 1 8.314 J K 1mol 1 760 o ln 358.9 K 85.8 C 1 298.15 K 42100 J mol 42.8 For 2-propanone 1 p R T2 ln 2 T1 H vap p1 1 1 1 8.314 J K 1mol 1 760 o ln 329.8 K 56.5 C 1 31900 J mol 221.0 298.15 K 5 B4. Referring to the phase diagram below for Xenon, answer the following questions: Xenon P, atm 59.0 1.0 0.37 A -121 -112 -108 +17 T, oC (a) The normal melting temperature of Xenon is:__-112___________oC (b) The normal boiling temperature of Xenon is:___-108__________oC (c) It is not possible to liquefy Xenon above ___+17_____________oC (d) Explain step by step what will happen to some solid Xenon which is taken out of a freezer (at point A on the diagram) and placed on a table top at 25oC. The solid will warm to -112oC At -112oC, the solid will melt to the liquid The liquid will warm to -108oC At -108oC, the liquid will vaporize to the gas The gas will warm to 25oC (e) Is solid Xenon more or less dense than liquid Xe? How do you know? The solid is more dense than the liquid. The solid exists at higher pressure than the liquid as shown by the positive slope in the S-L equilibrium line. Things are more dense at higher pressures. (f) Dry ice is CO2(s) in equilibrium with CO2(g) at 1 atm. Is it possible to have Xenon “dry ice”? Why? No, this is not possible, because solid and gaseous Xenon can only be in equilibrium below the triple point pressure, i.e. below 0.37 atm. (g) Why do you think working with liquid Xenon at 1 atm pressure is difficult in the lab? The substance is a liquid at 1 atm only between -112 and -108oC. Below -112oC, it freezes and above -108oC, it vaporizes.