Nylon Investigation - Nstacommunities.org
advertisement

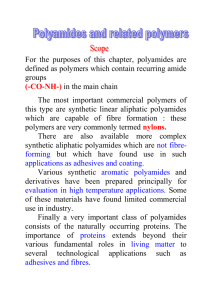
Nylon Investigation Subject Area: Chemistry Grade Level: High School Chemistry Lesson Title: Nylon Investigation National Science Standards: Science as Inquiry: 9–12 Physical Science Standards: Structure and Properties of Matter: 9–12 Chemical Reactions: 9–12 Suggested Prior Knowledge: concepts of polymerization, polymers Purpose: To understand how a polymer (Nylon) is made and to research the history of some polymers Key Vocabulary: monomer—individual repeating portion of a larger polymer molecule nylon—generic name for a family of polyamide polymers polymer—chemical compound formed from many bonded similar repeating units called monomers polymerization—process through which a polymer forms from the bonding together of monomers. There are many forms of polymerization and different systems exist to categorize them Objectives: 1. Students will be able to discuss the formation of nylon. 2. Students will investigate the physical properties of nylon. 3. Students will research the history of a polymer. Materials: - Safety goggles - Adipoyl chloride solution, 0.25M in hexane (10 ml/group) Nylon Investigation (High School Level) - Hexamethylenediamine solution, 0.50M in 0.50M NaOH (10 ml/group) - 50 ml beaker (one/group) - Paper clip - Water - Food coloring (red) - Paper towels Procedure: 1. At the 1939 World’s Fair, the DuPont company exhibited nylon, the world’s first synthetic polymer fiber. They touted the polymer as “strong as steel, as fine as a spider’s web, yet more elastic than any of the common natural fibers!” Discuss with students the natural and synthetic polymers with which they may be familiar. Discuss the polymerization reaction and how monomers are bonded together to form polymers. In the lab portion of this activity, students will make their own nylon and investigate its physical properties. 2. Students will follow up this lab experience with independent research into the history of other synthetic polymers, their production processes, and their uses. 3. Lab safety protocols should be followed. Goggles should be worn at all times. 4. Procedure: a. Each group will need 10 ml of each reagent, a paper clip, and a 50 ml beaker. b. Place the 10 ml of 0.25M adipoyl chloride solution (in hexane) into the beaker. c. Add 10 ml of the 0.50M hexamethylenediamine solution (in 0.50M NaOH) slowly, taking care not to allow the solutions to mix too much. This solution should be pink; if the color has faded add 1–2 drops of red food coloring. Do not stir or mix the solutions. The two solutions are immiscible (they will not mix). d. A white film will form at the interface of the two solutions. e. Use the paper clip, opened out to form a hook shape, to pull the nylon film out of the solutions. Pull the strands out slowly until all of the film is removed. f. Rinse the nylon several times with water and let dry on paper towels. 5. Once students have made their nylon, they can develop an investigation to learn about the physical properties of the nylon polymer. Encourage students to design a valid investigation that will allow them observe properties such as elasticity, strength, and flexibility. Some possible procedures include the following: Testing strength: hold each end of a strand of nylon and hang weights from the middle to determine what mass the nylon will hold. Testing elasticity: measure the length of a strand of nylon, then stretch the strand out as far as possible and measure the length of the stretched strand. Calculate the percent increase in the strand’s length. Nylon Investigation (High School Level) 6. After the students have finished their investigation, they should be able to describe both the chemical reaction they observed and the physical properties of the nylon they made in the lab. 7. Students may present their findings to their classmates and compare their results with those of their classmates. 8. Follow-up research into the composition and history of synthetic (or even natural) polymers is a great extension of this lesson. Additional Resources: http://www.flinnsci.com/Sections/Safety/safety.asp http://www.ehow.com/how_4451417_make-nylon.html http://inventors.about.com/od/nstartinventions/a/nylon.htm http://www.fibersource.com/F-TUTOR/history.htm http://en.wikipedia.org/wiki/Nylon Nylon Investigation (High School Level) Student Worksheet for Nylon Investigation Experiment Title: _____________________________Date: _______Name: __________ Student Hypothesis: _______________________________________________________ Materials: _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Procedure: Wear safety goggles for all lab work. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Nylon Investigation (High School Level) Data and Observations: Analysis of Data: _________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Nylon Investigation (High School Level) Conclusions: _____________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Nylon Investigation (High School Level)