CHM-101 Exam 4 Review
advertisement

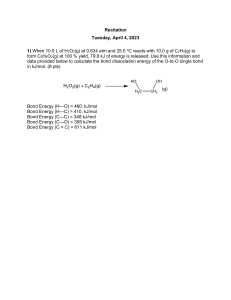
CHM-101 Exam 4 Review Review Materials: Chapters covered: 7.6-7.8, 8 (all, except section on dipole moment in 8.5 and section on formal charge in 8.6), 9.1-9.2. Online quizzes and practice tests: http://www.uncw.edu/chem/courses/Chm101/quizwww.htm Online practice quizzes that relate to this test: 4c Important Equations and Relationships from Chapters 7, 8, and 9: H°rxn = nD(broken) – nD(formed) Practice Questions: 1. Which one of the following has the largest radius? a) O2- b) F- c) S2- d) Cl- 2. Which of the following gives the correct order for atomic radius? a) b) c) d) Mg > Na > P > Si > Ar Ar > Si > P > Na > Mg Si > P > Ar > Na > Mg Na > Mg > Si > P > Ar 3. Of the following elements, which has the largest first ionization energy? a) Se b) As c) S d) Sb 4. Element M reacts with chlorine to form a compound with the formula MCl2. Element M is more reactive than magnesium and has a smaller radius than barium. Element M is a) Sr b) K c) Na d) Be 5. Which one of the following compounds produces an acidic solution when dissolved in water? a) SO2 b) Li2O c) CaO d) MgO 6. In which series are the ionic compounds arranged in order of increasing ionic bond strength? a) b) c) d) MgO < CaO < SrO LiCl < NaCl < NaF RbCl < SrCl2 < MgCl2 CaO < SrO < ScN 7. Which one of the bonds below is most polar? a) O-F b) O-N c) O-C d) O-B 8. Compared to a carbon-carbon double bond (C=C), a carbon-carbon single bond (C-C) is a) shorter and weaker. b) shorter and stronger. c) longer and weaker. d) longer and stronger. 9. How many electrons are shared in a double bond? a) 1 b) 2 c) 3 d) 4 10. Which one of the following represents the correct Lewis structure for IO3-? 11. Which one of the following violates the octet rule? a) OH b) PCl3 c) SF3+ d) CF3- 12. Which one of the following molecules or ions exhibits resonance? a) CO2 c) NH4+ b) CCl4 d) H3O+ 13. Using the table of bond enthalpies below, estimate the value of H for the following gas phase reaction. 2 NCl3 Bond N-Cl N N Cl-Cl a) -467 kJ/mol N2 + 3 Cl2 Average Bond Enthalpy (kJ/mol) 200 941 242 b) -583 kJ/mol c) -1067 kJ/mol d) 467 kJ/mol 14. What is the molecular geometry of the ion shown below? a) linear b) angular c) tetrahedral d) trigonal pyramidal 15. The approximate F-N-F bond angle in the molecule shown below is a) 90º b) 120º c) 109º d) 180º