4D traction force microscopy reveals asymmetric
advertisement

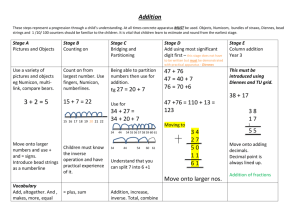
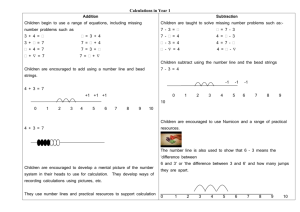
Supporting Information 4D traction force microscopy reveals asymmetric cortical forces in migrating Dictyostelium cells Delanoë-Ayari, H. and Rieu, JP Université de Lyon, F-69000, France; Univ. Lyon 1, Laboratoire PMCN; CNRS, UMR 5586; F-69622 Villeurbanne Cedex, France Sano, M. Department of Physics, University of Tokyo, Tokyo 113-0033, Japan In this supplement, we provide a detailed Materials and Methods section, two tests of the reconstruction method, and we present the 3D displacement map of a fibroblast cell. 1. Materials and Methods Cell culture and developmental conditions All experiments were conducted with AX-2 wild-type Dictyostelium discoideum cells at 21°C in the vegetative state. Cells were cultivated in petri dishes (88 mm diameter) to densities of ~5×106 cells/ml in HL5 media. They were washed twice in Phosphate Development Buffer (PDB: KH2PO4, 5mM; Na2HPO4-12H2O, 5mM; 2mMMgSO4 2mM; CaCl2 0.2mM; pH 6.9) and a droplet of 200 l at 5 x104 cells/ml in growth medium was deposited on the 22 mm circular elastomer layer. Polyacrylamide Gels Flexible polyacrylamide gel sheets were prepared and calibrated, as described previously [1], with 2.2% acrylamide, 0.25% bis, ammonium persulfate (10% w/v solution, 1:125 volume), TEMED (1:1250 volume; all Bio-Rad products) and 0.22% v/v of 0.2-μm fluorescent beads (2% solid green beads, Molecular Probes). Their elastic modulus was measured as previously described [1] at E=400100 Pa while the Poisson ratio was always taken as 0.5 [2, 3]. Cell speed Cell speed is calculated as the 2D cell mass center displacement during 8.5 sec. For this short timescale, it is a measurement of the cell protrusion activity. Detection of 4D substrate deformations We used a confocal microscope (Leica TCS-SP5) to detect bead displacements in xyzt dimensions (4D). We recorded simultaneously the cell shape at about +1 m from the elastomer surface (transmission channel) and a stack of fluorescence images every 0.35µm between z=0 and z=-6 m. In a continuous recording mode, at (128x128) pixels image size, the time lapse time step between two stacks was 8.5 sec, and xyz time series were recorded during about 7 min. At the end of that period, the undisturbed bead reference stack could be recorded, either because cells escaped the recorded field of view, or because cells were gently detached by adding a buffer droplet above the sample. Stacks where aligned at the pixel resolution in the x,y and z direction using image correlations with Matlab software. To measure the three dimensional bead displacements from the reference stack, we used the 3D tracking code created by John C. Crocker and Eric R. Weeks using the feature3D algorithm on IDL software [4]. To obtain a high 3D spatial resolution, we used a high density of beads. Because the bead displacement was quite greater than the bead to bead average distance we have first to calculate the rough displacements in the xy and z plane and correct for it before applying the tracking 3D algorithms. For the xy directions, we used the same 2D Particle Image Velocimetry (PIV) algorithm as described in [1]. It was performed on the first plane of the 3D stacks. For the z displacement, after obtaining the 3D positions of all the fluorescent beads, we calculated on each point of the 2D grid used for PIV, the difference in the z position of the higher beads observed in the stack compared to the reference one. After running the 3D tracking algorithm, displacement data were filtered using a median 3D filter to remove most of the eventually bad tracked particles. The code used will be freely available on simple request to the authors. Calculating the 3D force field: In the framework of linear elasticity theory [2], the deformation field u (r ) inside a semiinfinite elastic medium caused by a distribution of forces F (r ) on the surface is described by a Fredholm integral equation of the first kind, ui (r ) dr Gij (r r ) F j (r ) ui(r), where i and j refer to the x , y , z vectors components. Gij is the Green function of the elastic isotropic halfspace, which was calculated in the 19th century by Boussinesq [5]. Inverting the Fredholm integral to recover the force field is an ill-posed problem and requires some regularization techniques for a usual noise level on the recorded displacement field [6]. Our algorithm is largely inspired from the one of Schwarz et al [7] using the package of Matlab routines Regularization Tools by P. C. Hansen (http://www.netlib.org/) except it approximates the cellular force pattern by an ensemble of localized point-like forces on a triangular mesh generated under the cell by a Delaunay triangulation [1]. The only change with our previous work is that the inversion takes into account all components of displacements and forces vectors and not only tangential ones. We want to point out that contrary to some other works [2], we did not use a continuous representation of the stress tensor. Even, if we recognize the inherent disadvantages pointed out by Sabass et al. [8], we believe that this non continuous representation is useful for calculating the force field generated by vegetative dictyostelium cells with a characteristic force map similar to a contractile ring. To show the robustness of our method, we used simulations showing that we were able to correctly recover the value of the surface tension generated by a drop on a soft surface, and we compared the measured 3D force field exerted by a steel bead lying on a soft substrate to JKR theory. 2. Test of the reconstruction method simulating a drop lying on a soft substrate We tested our method of 3D force reconstruction with a simulated capillary drop deforming a soft substrate. First, we calculate the substrate z-deformation due to capillary forces (Laplace pressure and contact line forces) using the analytical formula calculated by Rusanov [9] for a liquid of surface tension =0.2*10-3N/m and contact angle with the substrate =36°. Fig 1A shows this calculated profile in red for a Young modulus of 400 Pa and poisson modulus of 0.5. Second, we used the plane z=0 and calculated for 500 different points, whose coordinates where chosen randomly, the value of the z deformations. We then got back the vertical forces using our algorithms. The force map is presented in Fig. 1B. Positive forces are well localized on the side of the drop while negative forces (due to Laplace pressure) are located everywhere under the drop. Third, the value of the surface tension, , can be then computed : F contour L * sin( ) , where L is the perimeter of the cell. The value of the surface tension obtained from this reconstruction was : 0.14 *10-3N /m, i.e, 30% lower than the initial value, but this is inherent to this kind of force reconstruction method ( see [8]). 0.12 0.1 0.08 0.06 Drop 0.04 0.02 Gel 2 µm 0 -0.02 -0.04 A B -0.06 Fig. 1. A) In red, profile of a drop lying on a soft gel, the scales in x and z are the same. B) Reconstructed z forces values obtained on a triangular mesh under the drop (values are in nN/µm). 3. Test of the reconstruction method using a steel bead lying on a soft polyacrylamide substrate We have also tested our reconstruction method using a steel bead (400 µm in diameter) lying on a soft substrate with fluorescent beads (400 nm in diameter) embedded inside (Fig 2). A stack of fluorescence images every 1 µm between z=+5 and z=-20 m was taken with a confocal microscope (20x magnification). In Fig. 2A-C, we show three different planes around the estimated elastomer top surface z=0. At z=+2µm (i.e., the focal plane is above the elastomer surface), most beads are out of focus except a ring of beads lifted up under the steel bead (Fig. 2A). The radius of this ring is about 60 µm. At z=0 µm, all fluorescent beads are in focus except under the steel bead adhesion zone where a black hole is visible (Fig. 2B). At z=-8 µm, the black hole under the adhesion zone has disappeared (Fig. 2C). To measure the three dimensional bead displacements from the reference stack, we used our 3D tracking code. The reference stack was taken after removing the steel bead with a strong magnet. In Fig. 2D, we show the upper surface bead displacement in the (r,z) plane averaged over all angles. From the bead deformation field, it is now possible to run our 3D force reconstruction code to get the 3D force field. For that, we used a mesh under a circle of radius a=60 µm centered under the steel bead. The pressure under the area of contact is displayed in Fig 2. E. We interpolated this pressure field on a polar grid (centered under the steel bead) so as to be able to calculate the mean pressure field under the steel bead as a function of the radius (Fig 2. F). In order to check the validity of our reconstruction technique, we tried first to use a simple Hertz modeling of contact, as was done in several studies [10]. However, the ring of fluorescent beads which is lifted up (Fig. 2A) cannot be described by the simple Hertz theory. Here, adhesion forces cannot be neglected. So we went on using the Johnson -Kendall –Roberts (JKR) [11]. This modeling is appropriate in presence of adhesion between the two bodies in contact and when the resulting elastic deformations (i.e., here several µm) are much larger than the range of adhesion forces (i.e., a molecular length). From it, one can calculate the pressure in the contact zone: 1/2 r2 p (r ) p0 1 2 a r2 p0 1 2 a 1/2 (1) where a is the radius of the contact zone between the steel bead and the gel, p0 2aE * ,with R R 1/2 2WE * E the radius of the steel bead and E and p0 a 1 2 , * , with W being the adhesion energy between the steel bead and the gel. The total load and the maximum displacement in the z direction obtained under the center of the steel bead can be calculated using: a 2 ( p0 2 p0 ) (3) P p0 2 p0 a 2 (2) and 2E* 3 In our case, the total load is given by the weight of the steel bead corrected for the buoyancy (i.e., P=2.1*10-6 N), and the maximum indentation is measured: 6 m . From equations (2) and (3), one can get the value of a and W by using the known value of elastic parameters: E =9700 Pa as measured by the cylindrical tube traction assay [1]), and =0.5. Here we obtained a=52 µm, which corresponds well to the radius of the ring of fluorescent cells in Fig. 2A, and W=10 mN/m. The values of the two pressures of Eq. 1 are p0=2.12 nN/m2 and p0’=-0.70 nN/m2. From these values, it is now possible to compare the pressure under the contact zone predicted by the JKR theory (Eq. 1) to our measurement using the flexible substrate method and fluorescent beads. The agreement displayed in Fig. 2F is very satisfactory without using adjustable parameters as p0 and p0’are obtained from the total load and the measured indentation. Fig 2. A B C) Fluorescent images of beads embedded in a polyacrylamide gel with a steel bead lying on the top of it. Images are taken at different depth in z A) z=+2µm . One can see a ring of beads of radius a. B) z=0 µm. All fluorescent beads are in focus except under the steel bead adhesion zone. C) z=-8 µm. The black hole under the adhesion zone has disappeared. D) Upper surface bead displacement in the (r,z) plane. It is a mean on all the angles which has been represented here. E) Pressure field obtained from the force reconstruction interpolated on a polar grid. D) Mean pressure above the steel bead as a function of the radius (data= blue stars, in red curve drawn from the JKR model (see equation 1)). 4. 3D deformation map of a fibroblast Our method can be used for different kind of cells. We present here the result showing the displacement field due to a fibroblast exerting forces on the substrate. Fig. 3. A) Measured in-plane projection of the 3D displacement field obtained from a NIH3T3 cell exerting forces on a 13kPa gel. (in red, vectors whose vertical component is directed upward and in green, vectors whose vertical component is directed downward). B) Upper surface bead displacement in a vertical plane along the blue line depicted in (A) References [1] H. Delanoë-Ayari, S. Iwaya, Y. T. Maeda, J. Inose, C. Rivière, M. Sano and J. P. Rieu, Cell Motil. Cytoskeleton 65, 314 (2008). [2] M. Dembo and Y. L. Wang, Biophys. J. 76, 2307 (1999). [3] T. Boudou, J. Ohayon, C. Picart and P. Tracqui, Biorheology 43, 721 (2006). [4] It can be downloaded at : http://www.physics.emory.edu/~weeks/idl/index.html) and translated for the Matlab software by Maria KilFoil (http://www.physics.mcgill.ca/~kilfoil/downloads.html). [5] L. D. Landau and E. M. Lifshitz, Theory of elasticity (Pergamon Press, Oxford, 1970), Vol. 7. [6] C. Barentin, Y. Sawada and J. Rieu, Eur. Biophys. J. 35, 328 (2006). [7] U. S. Schwarz, N. Q. Balaban, D. Riveline, A. Bershadsky, B. Geiger and S. A. Safran, Biophys. J. 83, 1380 (2002). [8] B. Sabass, M. L. Gardel, C. M. Waterman and U. S. Schwarz, Biophys. J. 94, 207 (2008). [9] A. Rusanov, Journal of Colloid and Interface Science 63, 330 (1978). [10] S. Munevar, Y. Wang and M. Dembo, Biophys. J. 80, 1744 (2001). [11] K. L. Johnson, Contact Mechanics (Cambridge University Press, 1985).