Solutions Problems:
advertisement
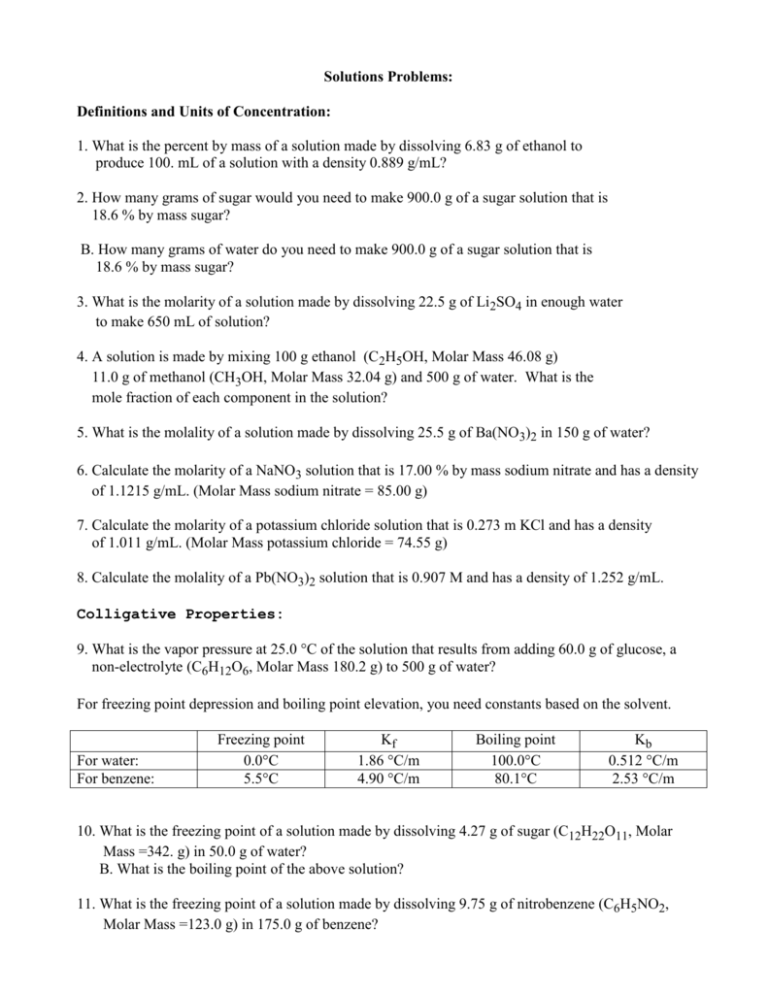
Solutions Problems: Definitions and Units of Concentration: 1. What is the percent by mass of a solution made by dissolving 6.83 g of ethanol to produce 100. mL of a solution with a density 0.889 g/mL? 2. How many grams of sugar would you need to make 900.0 g of a sugar solution that is 18.6 % by mass sugar? B. How many grams of water do you need to make 900.0 g of a sugar solution that is 18.6 % by mass sugar? 3. What is the molarity of a solution made by dissolving 22.5 g of Li2SO4 in enough water to make 650 mL of solution? 4. A solution is made by mixing 100 g ethanol (C2H5OH, Molar Mass 46.08 g) 11.0 g of methanol (CH3OH, Molar Mass 32.04 g) and 500 g of water. What is the mole fraction of each component in the solution? 5. What is the molality of a solution made by dissolving 25.5 g of Ba(NO3)2 in 150 g of water? 6. Calculate the molarity of a NaNO3 solution that is 17.00 % by mass sodium nitrate and has a density of 1.1215 g/mL. (Molar Mass sodium nitrate = 85.00 g) 7. Calculate the molarity of a potassium chloride solution that is 0.273 m KCl and has a density of 1.011 g/mL. (Molar Mass potassium chloride = 74.55 g) 8. Calculate the molality of a Pb(NO3)2 solution that is 0.907 M and has a density of 1.252 g/mL. Colligative Properties: 9. What is the vapor pressure at 25.0 °C of the solution that results from adding 60.0 g of glucose, a non-electrolyte (C6H12O6, Molar Mass 180.2 g) to 500 g of water? For freezing point depression and boiling point elevation, you need constants based on the solvent. For water: For benzene: Freezing point 0.0°C 5.5°C Kf 1.86 °C/m 4.90 °C/m Boiling point 100.0°C 80.1°C Kb 0.512 °C/m 2.53 °C/m 10. What is the freezing point of a solution made by dissolving 4.27 g of sugar (C12H22O11, Molar Mass =342. g) in 50.0 g of water? B. What is the boiling point of the above solution? 11. What is the freezing point of a solution made by dissolving 9.75 g of nitrobenzene (C6H5NO2, Molar Mass =123.0 g) in 175.0 g of benzene? 12. What is the molar mass (molecular weight) of a compound, if a solution made by dissolving 2.12 g of this compound in 48.92 g of water has a freezing point of – 0.679 °C?











