Supplementary Information (doc 528K)
advertisement

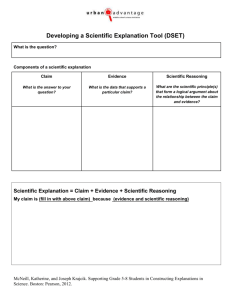
Supplemental Materials The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort Elise B. Robinson, ScD a,b,1, Andrew Kirby, BA a,b, Kosha Ruparel, MSE c, Jian Yang, PhDd, Lauren McGrath, PhD e, Verneri Anttila, PhD a,b,f, Benjamin M. Neale, PhD a,b, Kathleen Merikangas, PhD g, Thomas Lehner, PhD h, Patrick M.A. Sleiman, PhD i, Mark J. Daly, PhD a,b, Ruben Gur PhD c, Raquel Gur, MD, PhD c,, Hakon Hakonarson, MD, PhD i 1 For inquiries regarding this report: erobinson@atgu.mgh.harvard.edu a) Analytic and Translational Genetics Unit, Massachusetts General Hospital and Department of Medicine, Harvard Medical School, Boston, MA 02114. b) Stanley Center for Psychiatric Research and Medical and Population Genetics Program, Broad Institute of MIT and Harvard, Cambridge, MA 02142. c) Department of Psychiatry, Perelman of School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104. d) Queensland Brain Institute, University of Queensland, Brisbane, Australia. e) Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research and Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114. f) Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland. g) Genetic Epidemiology Research Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892. h) Office of Genomics Research Coordination, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892. i) Center for Applied Genomics, The Children’s Hospital of Philadelphia, Philadelphia, PA, 19104. Contents 1. 2. 3. 4. 5. 6. Summary of Computerized Neurocognitive Battery (CNB) Measures Genotyping and Imputation Genotypic Principal Components Analyses Unrotated Principal Components Analysis Common Factor and Rotated Principal Components Analyses Principal Component Loadings for Accuracy Traits; Phenotypic Correlation Matrix 7. Comparison of magnitude between phenotypic and genotypic correlations 8. Phenotypic and Genetic Association between Domains and Factor Scores; 9. Variation Explained by Genic and Intergenic SNPs 1) Summary of Computerized Neurocognitive Battery (CNB) Measures The CNB was developed by Ruben Gur, Raquel Gur and colleagues at the University of Pennsylvania. Its component measures are described in Gur et al. 2010 and 2012. The Wide Range Achievement Test (WRAT) is described by Wilkinson and Robertson (2006). Cronbach’s alpha was estimated based on the consistency of correct responses on each measure. Table S1. Reliability of CNB measures Trait Measure (1, 2) Abstraction and Mental Flexibility Attention Working Memory Facial Memory Spatial Memory Verbal Memory Language Reasoning Nonverbal Reasoning Spatial Reasoning Age Differentiation Emotional Differentiation Emotional Identification Wide Range Achievement Test Penn Conditional Exclusion Test Penn Continuous Performance Test Letter N-Back Penn Face Memory Test Visual Object Learning Test Penn Word Memory Test Penn Verbal Reasoning Test Penn Matrix Reasoning Test Penn Line Orientation Test Penn Age Differentiation Test Penn Emotion Differentiation Test Penn Emotion Identification Test Wide Range Achievement Test (3) Cronbach’s Alpha NA 0.94 0.87 0.68* 0.52 0.80 0.77* 0.86* 0.93* 0.78* 0.81 0.75* NA Note: NA=Not Applicable; Cronbach’s alpha was not calculated for abstraction and mental flexibility because the test is designed to elicit incorrect responses at various points, several stimuli are repeated throughout the test, and the length of the test varies between individuals based on their performance (2). The reading items from the WRAT were used in this analysis—see Robertson et al. for details and validation. Multiple, highly similar forms were used for several of the measures, indicated by asterisks following the reliability estimates. This approach was used to prevent learning effects in longitudinal (follow-up) research using the cohort. The reliability values with asterisks indicate an average Cronbach’s alpha reliability estimate weighted by the number of individuals who took each form of the test. No more than 4 forms were used for any individual measure and the range of the form-specific alphas did not exceed 0.12 per measure. 2) Genotyping and Imputation The data were cleaned and prepared for ancestry analysis at Massachusetts General Hospital. Beginning with the 5141 self-described white non-Hispanic individuals, for each chip array dataset, we: a) removed individuals with sex-mismatched genotype and phenotype data (n=85); b) removed SNPs with genotype missingness>5%, c) removed individuals with marker missingness>2% (total n=91), d) removed SNPs with missingness>1%, e) removed SNPs with empirical minor allele frequency greater or less than 0.15 from their HapMap CEU value, f) removed SNPs with Hardy-Weinberg Equilibrium test p-values<1x10-6, and g) removed individuals with heterozygosity values greater than 0.05 or less than -0.05 (total n=97). Using a linkage disequilibrium pruned subset (n=39,830 SNPs) of the remaining markers common to each of the four platforms (n= 236,473 SNPs), we identified and removed individuals with excess relatedness (pi_hat>0.1, n=309). We then conducted a principal components analysis as described in the Methods. The individuals included in the genetic analyses were selected through the steps above as well as further phenotypic analyses described in the Methods. Imputation The following data steps were implemented in a separate stage of the study at the Children’s Hospital of Philadelphia. Samples were genotyped on one of three Illumina arrays, the HumanHap550, HumanHap610, or the OmniExpress v2. For each chip type, we excluded from further analysis any samples that had missing genotypes for more than 2% of the SNPs on the array; further, we only included SNPs with genotype missing rates < 5%, minor allele frequency > 1%, as well as Hardy-Weinberg Equilibrium test P value > 0.0001. Duplicate samples and cryptic relatedness: we generated pairwise IBD values for all samples using the --genome command in PLINK(ref), excluding one sample from any pair with a PI_HAT value exceeding 0.3. PI_HAT values estimate the degree of genetic relatedness between individuals, where: >0.99 indicate sample duplication or monozygotic twins, 0.5 indicates dizygotic twins or regular siblings, and 0.25 indicates half siblings. Prephasing: for each of the three chip types, samples were prephased for imputation using the SHAPEIT (http://www.shapeit.fr) package. The GWAS SNPs were lifted over to hg19 build37 coordinates, marker strand alignments checked against the 1000 genomes Phase I reference alleles and any misaligned SNPs flipped prior to phasing. Each entire chromosome was prephased separately. As prephasing was carried out for each chip type separately, haplotypes were restricted to SNPs common to all arrays prior to imputation. Imputation: unobserved genotypes in each cohort were imputed using the IMPUTE2 package (http://mathgen.stats.ox.ac.uk/impute/impute_v2.html) and the reference haplotypes in Phase I of the 1000 genomes (June 2011 release). The used reference included 37,138,905 million variants from 1,094 individuals from Africa, Asia, Europe and the Americas. Genotype concordance: internal cross validation was carried out automatically by IMPUTE2. The calculation was performed by masking one variant at a time in the study data, imputing the masked variant and comparing the result to the original genotype. Average concordance for all datasets was > 90%. Post-imputation QC: Imputed data was QC'd using the qctool package (http://www.well.ox.ac.uk/~gav/qctool), SNPs with a maximum genotype probability of less than of 0.9 were excluded from further analysis, as were SNPs with a minor allele frequency below 1%. 3) Genotypic Principal Component Analyses The post-QC sample of unrelated individuals who identified themselves as white nonhispanic (WNH; n=4,559) is plotted in dark blue against the HapMap3 populations (northern and western Europe (CEU); Han Chinese in Beijing, China (CHB); Japanese (JPT); Yoruba (YRI); African ancestry in the southwestern USA (ASW); Chinese in Colorado, USA (CHD); Gujarati Indians in Texas, USA (GIH); Luhya in Webuye, Kenya (LWK); Maasai in Kinyawa, Kenya (MKK); Mexican ancestry in California, USA (MXL); and Tuscany, Italy (TSI)) (4). PC1, PC2, and PC3 indicate loadings onto the first, second, and third principal components respectively. Figure S1a. Full WNH sample PC1 v PC2 Figure S1b. Full WNH sample PC2 v PC3 After excluding outlying individuals, the reduced European-American sample below was carried through for phenotypic exclusions and analyses analyses (n=4,050). These remaining individuals are plotted in dark blue against the same HapMap3 populations. The final analytic sample (n=3,689) is described in Tables 4 and 5, below. Figure S1c. Reduced sample PC1 v PC2 Figure S1d. Reduced sample PC2 v PC3 4) Unrotated Principal Components Analysis An unrotated PCA was conducted to estimate the variance captured by the first principal component. There was a dominant first principal component that explained 27.7% of variation in the accuracy variables. Variable loadings onto the first principal component are presented in Table S2, below. These principal component scores were not used in the genetic analyses as the accuracy variables are highly correlated and unrotated principal components analysis does not yield correlated or interpretable additional components. Table S2. Unrotated principal components analysis PC1 Loading Abstraction and Mental Flexibility Attention 0.38782 Language Reasoning Nonverbal Reasoning Working Memory 0.67553 Spatial Reasoning 0.61953 WRAT 0.56751 Age Differentiation 0.54990 Emotion Identification Emotion Differentiation Facial Memory 0.32148 Spatial Memory 0.45616 Verbal Memory 0.37138 0.50369 0.63866 0.50393 0.59899 0.51301 Note: PC=Principal Component. 5) Common Factor and Rotated Principal Components Analyses The common factor analyses for the CNB (including the WRAT) variables showed a single strong, dominant factor. Rotation was not possible in this case as there was only one factor. The eigenvalue estimates reflect the variation explained by each factor relative to any single trait used in the factor analysis. For example, an eigenvalue of 1 indicates that the factor does not explain any more variation than a single component trait of the 13 accuracy traits. Figure S2a. Common factor analysis Figure S2b. Principal components analysis (promax rotation) We conducted additional genetic analyses using the principal component loadings. While the PCA similarly yielded a dominant first principal component, two additional principal components had eigenvalues exceeding one. Rotation allows the additional components to be interpreted. The first PC includes the reasoning and executive function items; the second the social cognition items; and the third the memory items. 6) Rotated Principal Components Analysis— Trait to Component Correlations We conducted the rotated PCA of the variables four times—first with the full analytic sample and then in each of the three age groups below. This table shows trait/principal component pairs with a correlation coefficient above 0.35. All variables were associated with only one principal component with a correlation above 0.35, except facial memory accuracy (FMEM_A; in addition to the correlation shown below, it showed a 0.43 correlation to PC2). Table S3a. Item to principal component correlations Variable Full Ages Cohort 8-11 Abstraction and 0.492 0.465 Mental Flexibility PC1 Attention 0.462 0.466 Loadings Language Reasoning 0.665 0.606 Nonverbal Reasoning 0.680 0.662 Working Memory 0.593 0.604 Spatial Reasoning 0.584 0.531 WRAT 0.562 0.473 PC2 Age Differentiation 0.772 0.800 Loadings Emotion Identification 0.537 0.354 Emotion Differentiation 0.715 0.714 PC3 Facial Memory 0.689 0.725 Loadings Spatial Memory 0.667 0.591 Verbal Memory 0.711 0.580 Ages 12-16 0.461 Ages 17-21 0.548 0.461 0.714 0.641 0.587 0.589 0.602 0.790 0.501 0.740 0.652 0.673 0.739 0.532 0.626 0.718 0.633 0.548 0.601 0.777 0.451 0.809 0.743 0.621 0.718 The factor structure and loadings were similar across age groups suggesting consistency in the phenotypic correlation structure. Table S3b. Phenotypic Correlation Matrix (See Phen_Corr_Matrix_Revised.xlsx) 7) Comparison of magnitude between phenotypic and genotypic correlations. Figure S3. Comparison of magnitude between phenotypic and genotypic correlations. Note: The diameter of the circles corresponds to the magnitude of the phenotypic and genotypic correlations between the domains (r=0-1). The magnitude of the genotypic correlation between emotional identification and nonverbal reasoning is not presented because it is less than 0. 8) Phenotypic and Genetic Association between Domains and Common Factor Score Table S4. Estimated phenotypic and genetic correlations between the common factor score and its component variables with nominally significant univariate heritability estimates. Correlation r(p) p(r(p)=0) r(g) (SE) p(r(g)=0) p(r(g)=1) Common Factor Score (CFS) – Domain CFS-Emotion Identification 0.272 <0.0001 0.184 (0.178) 0.2 1e-07 CFS-Verbal Memory 0.380 <0.0001 0.749 (0.174) 0.001 0.09 CFS-Language Reasoning 0.684 <0.0001 0.972 (0.082) 0.0004 0.4 CFS-Nonverbal Reasoning 0.636 <0.0001 0.686 (0.100) 0.0005 0.0003 CFS-Spatial Reasoning 0.594 <0.0001 0.738 (0.117) 0.0008 0.01 CFS-WRAT 0.566 <0.0001 0.743 (0.110) 0.0002 0.01 Note: r(p)= estimated phenotypic correlation; p(r(p)=0)= probability that phenotypic correlation=0; r(g)=estimated genotypic correlation; p(r(g)=0)= probability that genotypic correlation=0; p(r(g)=1)= probability that genotypic correlation=1; SE=standard error; WRAT=Wide Range Achievement Test. 9) Variation Explained by Genic and Intergenic SNPs Figure S5. Variation in complex cognition explained by SNPs within genic and intergenic regions of the genome. Note: error bars indicate one standard error; p values = probability of no joint association between the genic and intergenic regions and the common factor score. References 1. Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26(2):251-65. Epub 2012/01/19. 2. Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. Journal of neuroscience methods. 2010;187(2):254-62. Epub 2009/12/01. 3. Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 professional manual. Lutz, Florida: Psychological Assessment Resources; 2006. 4. Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52-8. Epub 2010/09/03.