PHARMACIST: Training Documentation for Clinical Trials
advertisement

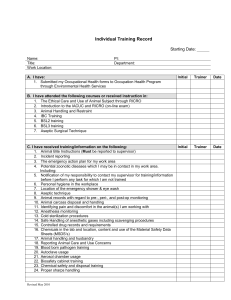
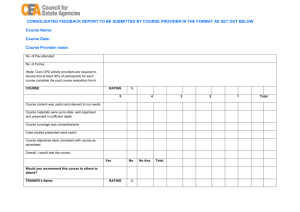
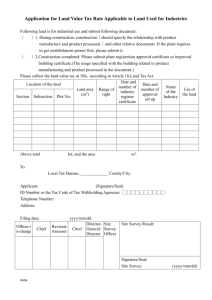
Personal Training Documentation Topic: Network of Networks (N2) Standard Operating Procedures (SOP) SOP# Version Title 002 Research Team Roles and Responsibilities 003 05 05 004 05 Clinical Research Protocol Feasibility and Site Selection 005 05 Study Initiation Activation 006 05 Informed Consent Forms 007 05 REB Submissions and Ongoing Communication 008 05 Informed Consent Process 009 05 Subject Recruitment and Screening 010 05 Management of Investigational Products 011 05 Management of Biological Specimens 012 05 SAR Reporting in Clinical Trials 013 05 Study Monitoring and Communication 014 05 Clinical Data Management 015 05 Investigator Study Files and Essential Documents 016 05 Study Close-Out 017 05 Audits and Inspections 018 05 Clinical Trial Application 019 05 Confidentiality and Privacy 023 01 Clinical Trial Application (Natural Health Products) Investigational Testing Authorization (ITA) for Medical Devices (non-IVDD) and Manufacturer/Sponsor Obligations Equipment Calibration and Maintenance 024 025 01 01 Research Team Training Investigator Initiated and Electronic Data management SOPs 100 03 CRF Design 101 03 Study Analysis and Reporting Page 1 of 3 Version 2 102 03 Protocol Development 103 02 Data Management Plan 104 Database Setup 105 02 02 106 02 File Transfer 107 02 Database Lock and Archiving 108 02 System Setup Maintenance and Security 109 02 System Backup and Recovery Database Maintenance The trainer(s) listed below is/are available to answer your questions. The above-named N2 SOPs and corresponding quiz questions have been reviewed and understood by the listed employee. The signature and date below is their attestation of completion of training. Training was overseen by _______________ Employee Signature: Date: (yyyy/mmm/dd) Print Name: Title: Dept/Prog: Trainer Signature: Date*: (yyyy/mmm/dd) Print Name: Title: Dept/Prog: *Denotes the date that the listed trainer has determined that the listed employee is appropriately trained on the Topic. Page 2 of 3 Version 2 NOTE: Trainer(s) to maintain all communications related to training (i.e. emails and attachments, posters, etc.) to confirm the method of training, documents circulated for review and duration of training, as applicable.