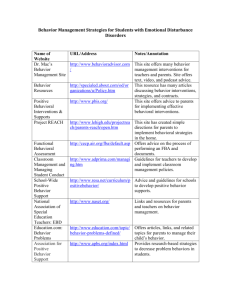
Principal components analysis (PCA): For the analysis, we introduce
advertisement

Principal components analysis (PCA): For the analysis, we introduce the following notation. Let Jt , i; , s be the current density in voxel i, as estimated by LORETA, in condition at t time-frames after stimulus onset for subject s. Let area :Voxel fBA be a function, which assigns to each voxel i Voxel the corresponding fBA b fBA. In a first pre-processing step, we calculate for each subject s the value of the current density averaged over each fBA (1) xt , b; , s 1 Nb J t , i; , s ib where Nb is the number of voxels in the fBA b, in condition for subject s. In the second analysis stage, the mean current density x t,b;,s from each fBA b, for every subject s and condition, was subjected to spatial PCA analysis of the correlation matrix and varimax rotation In the present study the spatial PCA uses the above-defined fBAs as variables sampled along the time epoch for which EEG has been sampled (0-1000ms; 512 time-frames), and the inverse solution was estimated. Spatial matrices (each matrix was sized b x t = 36 × 512 elements) for every subject and condition were collected, and subjected to PCA analyses, including the calculation of the covariance matrix; eigenvalue decomposition and varimax rotation, in order to maximize factor loadings. In other words, in the spatial PCA analysis we approximate the mean current density for each subject in each condition as (2) xt; , s x 0 , s ck (t )x k , s , k where here xt;,s R36 is a vector, which denotes the time-dependent activation of the fBAs, x 0 ,s is their mean activation, and x k ,s and c k are the principal components and corresponding coefficients (factor loadings) as computed using the principal component their analysis. References: (1) Arzouan Y, Goldstein A, Faust M. Brainwaves are stethoscopes: ERP correlates of novel metaphor comprehension. Brain Res 2007; 1160: 69-81. (2) Arzouan Y, Goldstein A, Faust M. Dynamics of hemispheric activity during metaphor comprehension: electrophysiological measures. NeuroImage 2007; 36: 222-231. (3) Chapman RM, McCrary JW. EP component identification and measurement by principal components analysis. Brain and cognition 1995; 27: 288-310. (4) Dien J, Frishkoff GA, Cerbone A, Tucker DM. Parametric analysis of event-related potentials in semantic comprehension: evidence for parallel brain mechanisms. Brain research 2003; 15: 137-153. (5) Dien J, Frishkoff GA. Principal components analysis of event-related potential datasets. . In: Handy T (ed). Event-Related Potentials: A Methods Handbook. Cambridge, Mass MIT Press; 2004. (6) Potts GF, Dien J, Hartry-Speiser AL, McDougal LM, Tucker DM. Dense sensor array topography of the event-related potential to task-relevant auditory stimuli. Electroencephalography and clinical neurophysiology 1998; 106: 444-456. (7) Rosler F, Manzey D. Principal components and varimax-rotated components in eventrelated potential research: some remarks on their interpretation. Biological psychology 1981; 13: 3-26. (8) Ruchkin DS, McCalley MG, Glaser EM. Event related potentials and time estimation. Psychophysiology 1977; 14: 451-455. (9) Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology 2001; 38: 343-358. (10) Squires KC, Squires NK, Hillyard SA. Decision-related cortical potentials during an auditory signal detection task with cued observation intervals. Journal of experimental psychology 1975; 1: 268-279. (11) van Boxtel A, Boelhouwer AJ, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology 1998; 35: 690-697.





![See our handout on Classroom Access Personnel [doc]](http://s3.studylib.net/store/data/007033314_1-354ad15753436b5c05a8b4105c194a96-300x300.png)