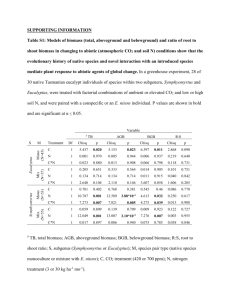
Positive scaling of mass-density (M
advertisement

1 Positive scaling of mass-density (M-N) relationship under harsh environments Bing-Ru Liu1, Dong-Liang Cheng2, Gen-Xuan Wang3 2 3 (1 Key Laboratory of Arid and Grassland Agroecology at Lanzhou University, Ministry of 4 Education, Lanzhou 730000, China; 5 2College of Geographical Science, Fujian Normal University, Fuzhou 350007, China; 3College 6 of life science, Zhejiang University, Hangzhou 310029, China) 7 Author 8 Tel: +86 (0)571 8697 1083; for correspondence: 9 Fax: +86 (0)571 8697 1083 10 Email: wanggx@zju.edu.cn 11 *Supported by the National Natural Science Foundation of China (90102015, 30170161). 12 Received: 7 Jan. 2007 13 Accepted: 23 Apr. 2007- 14 Handling editor: Da-Yong Zhang 15 16 17 18 19 20 21 22 23 24 25 1 1 Abstract 2 Relationship between body size and population density is one of the most 3 fundamental aspects of population biology. Traditional studies indicate that plant 4 biomass (M) is often negatively related with density (N), which is based on 5 competition among plant individuals, and to some extent, is suitable to explain plant 6 dynamics in benign situations. However, it fails to explain in harsh environments. We 7 used the published data of Usoltsev (2001) and Bondarev (1997), as well as the data 8 we collected in Minqin, northwest of China, to test the M-N relationship under hash 9 environments. The results indicated that the value of the scaling exponent for the 10 aboveground biomass vs. density was 1.19 for Larix gmelinii in Central Siberia, and 11 1.87 for Artemisia arenaria in Minqin. The data showed that positive scaling 12 exponents were yielded under harsh environment, and decreased with the 13 improvement of abiotic conditions. It implied that the positive interactions among 14 individuals might improve the utility efficiency of space. It also indicated that the 15 energetic equivalence rule (EER) was not applicable to all species under all growth 16 conditions. Furthermore, new and appropriate approaches should be developed to 17 investigate M-N relationship under different limited resources or stress conditions, in 18 order to properly understand M-N relationship. 19 20 21 Key words Energy equivalence rule, environmental gradient, harsh environment, mass-density relationship, positive interactions, positive scaling exponents. 22 23 24 25 26 27 2 1 Relationship between body mass and density is one of the most fundamental 2 aspects of population biology, and it has major implications for the structure of 3 ecological communities (Brown 1995; Damuth 1998). In an even aged monospecific 4 plant population, when M (biomass) and N (density) are assumed to represent two 5 variables, respectively, this relationship is usually modeled as: 6 M=KNλ or logM = logK +λlogN 7 Where M and N are the average weight and real density of surviving individuals, 8 respectively, K and λ are the constant and the scaling exponent (slope of M-N 9 relationship), respectively (Yoda et al. 1963). If averaged body mass per plant plotted 10 against density, this relationship becomes: m = kN γ, where the scaling exponent, γ = 11 (λ - 1). 12 Among a series of studies of M-N relationship for 30 years, one of controversial 13 topics was the value of λ. Some workers proposed that λ was invariable regardless the 14 changing of abiotic conditions (White and Harper 1970). Theoretical justifications of 15 self-thinning have been reported that the classical exponent equals to -1/2 for plants 16 was suggested in a crowded monoculture (Yoda et al. 1963; Westoby 1984; White 17 1985; Lonsdale 1990; Yastrebov 1996) or mixed stand (Westoby 1984). The usual 18 explanation of self-thinning law has been largely based on geometric properties of 19 individual plant (Yoda et al. 1963; Westoby 1984; White 1985; Lonsdale 1990), and it 20 was thought that a slope (λ) of −1/2 applied to all of the species within the plant 21 kingdom (the intercept value, log K, was less clear)( Scrosati 2005). Theoretically, the 22 previous prediction of a universal slope of −1/2 was based on the assumption that 23 plants maintain the same shape (isometric growth) during self-thinning, which is now 24 known to be unrealistic (Weller 1987b), therefore, its validity is still in doubt (Weller 25 1987a; Zeide 1987). Hence, dynamic M-N relationships should be calculated (when in 26 the linearized, log-log form) through a Model II regression technique (such as 27 principal components analysis or reduced major axis), since the Model I regression 28 technique of least squares linear regression should not be used for this purpose, which 29 assumes that the X variable (N, in this case) should be fixed (Weller 1987a; Sokal and 30 Rohlf 1995).The shallower (than −1/2) nature of the interspecific biomass-density 3 1 slope is independently supported by a theoretical model based on trends in plant 2 geometry across the plant kingdom (Weller 1989). More recent theoretical models 3 based on morphological and physiological considerations specifically predict a slope 4 of −1/3 for the IBDR (Enquist et al. 1998; Franco and Kelly 1998), which coincident 5 with Weller’s (1989) empiric findings. And some authors proposed that the exponent 6 (λ) was ought to be -1/3 for all plant species, expressing as M∝N-1/3 (West et al. 1999; 7 Brown et al. 2004). This value is based on the fractal-like construction of internal 8 resource distribution networks (Enquist et al. 1998; West et al. 1999). In contrast, a 9 number of investigations reported that λ is closely depended on usually regulated by 10 abiotic or biological factors (Yoda et al. 1963; Weller 1987a; White and Harper 1970; 11 Ford 1975; Osawa 1995). Some abiotic factors, such as light, water, nutrition and 12 temperature, can affect the λ directly (Thomas and Weiner 1989; Morris 1999; 13 Callaway). Leaf area also intensively influenced λ, which varied around -1/2 (Osawa 14 and Allen 1993; Osawa 1995), and increased λ is also observed when the light is 15 weakened (Hiroi and Monsi 1966; Dunn and Sharitz 1990), or the provision of 16 nutrition is decreased (Zeide 1987; Morris 2003). In a word, the variation of λ can be 17 explained by the argument that intensity of competition changed along with the 18 changing resource level (Morris 1999). 19 It is generally believed that M-N relationship occurs and appears to be the 20 consequence of negative interactions between individuals, i.e. competition for space, 21 and λ will change with the variation of interaction among plant individuals (Morris 22 and Myerscough 1991; Morris 1999). More recently, experimental studies have 23 discovered that plant interaction may vary along the environmental gradient, and 24 positive effects can emerge among plant individuals under stressful environments 25 (Bertness and Callaway 1994; Callaway and Walker 1997). Positive interactions, or 26 facilitation, occur when one individual ameliorates stressful abiotic or biotic 27 conditions for another (Bruno and Bertness 2001). Field investigations from a wide 28 variety of habitats not only have demonstrated the strong effect of facilitation on 29 individual fitness, population distributions and growth rates, species composition and 30 diversity, and even landscape-scale community dynamics (Callaway 1995; Bruno and 4 1 Bertness 2001), but also have indicated that positive interactions are likely to be as 2 ubiquitous as competitive interactions (Callaway 1995; Hector et al. 1999; Tewksbury 3 and Lloyd 2001). Bertness and Callaway (1994) hypothesized that the importance of 4 facilitation in plant organization increased with abiotic stress while the relative 5 importance of competition decreased. Complex combination of the effects of 6 competition and facilitation operating simultaneously among plant species appears to 7 be the rule in nature (Pugnaire and Luque 2001). Obviously, M-N relationship based 8 on competition seems to be suitable to explain plant dynamics in benign situations, 9 but when it comes to plants in harsh environments, it will fail to do that. 10 In physically harsh environments such as salt marsh, desert, and alpine habitats, 11 where resource is inadequate, benefits provided by a tougher neighbor may be more 12 likely to favor growth than competition with that tough neighbor is likely to reduce 13 growth. The net effect of plant interactions is frequently measured as the ratio of some 14 performance variables, usually the ratio biomass between individuals with and 15 without removing their neighboring plants. The relative interaction index (RII), one 16 way to describe the net effect of plant interaction, is expressed in the following 17 equation (Armas et al. 2004): 18 RII = (Bw-B0 ) / (Bw+B0 ) 19 Where Bw (high density) is the mass of plants with neighbors and Bo (low density) 20 is the mass of isolated individuals. RII has defined limits [−1, +1], it is symmetrical 21 around zero with identical absolute values for competition and facilitation, 22 Competition prevails when 0≤Bw< B0< +∝ while facilitation prevails when 0≤B0 < 23 Bw < +∝, when there is no interaction or the outcome is neutrals, Bw= B0. Evidently, 24 RII > 0 indicated the positive interactions and positive relationship between body 25 mass and density. Presumably then, the scaling exponent of M-N relationship will 26 change with abiotic gradient, and the positive value would appear under the stressful 27 situations. 28 In this paper, we prediction that the λ is positive under harsh environments by 29 establishment the M-N relationships of Gmelin larch (Larix gmelinii (Rupr.)) forests 30 and desert shrub species (Artemisia arenaria DC.), which are all natural communities 5 1 growing under extremely harsh environments, and the scaling exponent in plant 2 communities varied along environmental gradients. 3 4 Results 5 6 The relationship between aboveground biomass and population density for two 7 natural populations were shown in Fig. 1. For L. gmelinii (Fig. 1A), the regression 8 equations shown are logM = 4.36 + 1.19 logN. The RMA regression indicated a 9 scaling exponent of 1.19 with 95% confidence interval (CI,0.96-1.51) and an 10 intercept 4.36 with 95% CI (4.11-4.78) in the poorest site Va-Vb(●) (R2=0.51, 11 P<0.0001). While the scaling exponent was zero at site index V(×) and I-IV(△). 12 For A arenaria (Fig. 1B), the regression equations shown is logM = 2.30 + 1.87 13 logN. It indicated a scaling exponent of 1.87 with 95% CI (1.60 to 2.23) (P < 0.0001; 14 R2=0.60). 15 16 Discussions 17 18 Trees provide an ideal opportunity to test the plant M-N relationship and energy 19 equivalence rule for the plant communities (Niklas et al. 2003). Data for L. gmelinii 20 growing in permafrost soil of Central Siberia were anomalous in having positive λ for 21 the aboveground biomass and density (Fig. 1A). The data in which biomass decreased 22 as density declines have been observed before, but dismissed as the results of 23 catastrophic events, such as lodging of surviving plants, or pathogenic epidemics, 24 both of which are examples of substantial density-independent mortality (Westoby 25 1984). Neither lodging nor extensive pathogenic attack occurred in our studies. In 26 addition, competition-density effect (C-D effect) (Kira et al. 1953; Hagihara 1999) 27 also suggests a positive scaling. When population density was the only variable, and 28 all other factors were held constant experimentally, nearly all populations followed 29 the relationship M=kNλ, where λ varied between 0 and 1. Although C-D effect has the 30 same mathematical expression as M-N relationship, it presents different ecological 6 1 meaning. C-D effect describes a temporal variation of density during the growth until 2 the appearance of density-dependent mortality; M-N relationship shows a spatial 3 variation of density at mature populations once mortality commences. L. gmelinii 4 experienced dramatically density-dependent mortality in population growth. For 5 example, population density varied from more than 200 no. /m2 for juveniles to less 6 than 0.4 no./m2 for mature stands. More important, scaling exponents of L. gmelinii 7 was different with other studies carried out in trees-dominated communities. 8 Numerous studies have shown that the basic allometric relationships, aboveground 9 biomass scaled as the -1/3 power of stand density, surprisingly varied slightly with 10 species diversity, total standing biomass, latitude and geographic sampling in area 11 (Enquist et al. 1999; Whittaker 1999; Enquist and Niklas 2001). Therefore, it is not 12 surprised that positive scaling might demonstrate positive interactions among 13 individuals of L. gmelinii. It’s assumed that this positive relationship between body 14 mass and density is based on resource limitations. As conceptualized by Callaway et 15 al. (2002) for shifts in positive interactions on elevation gradient, we believe that 16 temperature and nitrogen content in soil are less limiting to plant growth at benign 17 environments, but continuous permafrost temperature and lower nitrogen limit plant 18 growth. Amelioration of these severe stresses via facilitation by neighboring 19 individuals indicated a positive relationship, rather than competition for shared 20 resource, which resulted in a negative relationship. 21 This positive pattern of M-N relationship should be common not only in permafrost 22 but also under other stressful environments. A. arenaria in desert of northwest China 23 indicated that λ is 1.87, which is higher than the boundary of C-D effect (Fig. 1B). 24 This may be likely caused by the positive interactions in relatively high density. The 25 physical stress of desert plant communities is severe and stress gradients arise with 26 variation in water availability or fertility (Tewksbury and Lloyd 2001). Accordingly, 27 positive interactions are thought to be of great importance in arid and semi-arid areas 28 (Whitford 2002). Tirado and Pugnaire (2003) pointed out that high adult plant density 29 would significantly increased plant product, such as flower and fruit, and showed a 30 higher mass of seeds as a result of enrichment in patches. In addition, plants showed 7 1 an increasing aboveground biomass through aggregation (Stoll and Prati 2001), and a 2 positive feedback between body mass and soil water (Wilson and Agnew 1992). The 3 more soil water the faster the plant growth, and the larger the plant the more soil 4 water available to it. 5 How positive interactions induce positive scaling? We hypothesized that the 6 possible mechanism was yielded by the spatial utility. In a crowded population under 7 benign environment, the aboveground competition for space was sufficient enough to 8 maintain a constant leaf area index (LAI), which resulted in a negative relationship 9 between body mass and density (Hutchings and Budd 1981; Osawa and Allen 1993). 10 However, when the limited resource was not space but nutrients, moisture or 11 temperature, the LAI changed as resource level varied, which led to the deviation 12 from a slope of -1/2 (Hamilton et al. 1995). Under stressful environments (e.g. our 13 study sites), space was not fully occupied by plants, and the surplus space did not 14 contribute to the accumulation of aboveground biomass. The positive interactions 15 among individuals might improve the utilization of space, which will lead to a 16 positive M-N relationship. 17 The scaling exponent of M-N relationship is often closely related with the energetic 18 equivalence rule (EER). This rule states that the amount of energy that each 19 population uses per unit of area is independent of its mean body size. This is a 20 consequence of estimating an empirical slope of –3/4 for the relationship between 21 density and mean mass per plant (m), and a slope of 3/4 for the relationship between 22 metabolic requirement (B) and m (Enquist et al. 1998), combining the two allometric 23 equations results in a zero exponent of population energy use (PEU) in relation to m. 24 Since depending on the λ = -1/3, EER can be tested by the scaling exponent of M-N 25 relationship. Although it has been shown to hold at local, regional and worldwide 26 scales for tree-dominated communities, and has been hypothesized to emerge from the 27 allometric rules that influence the behavior of individual plant species competing for 28 space and limiting resources (Enquist and Niklas 2001), it is important to note that 29 EER does not imply that PEU of a pure species is invariable with body mass, in part 30 because the mean for MS-1/3 (MS: multi-species) does not equal SS-1/3 (SS: 8 1 single-species). Firstly, some workers investigating in forest indicated that λ varied 2 around -1/2 (Osawa and Allen 1993; Osawa 1995). In a forest stand thinning along a 3 slope of -1/2, Ford (1975) observed that the dominant trees showed a slope of -0.9. 4 Secondly, Carbone and Gittleman (2002) predicted that variation in local resource 5 supply rate naturally limited population density and may alter expected scaling pattern. 6 It was tested that the same species on the sites of different resource levels would lead 7 to apparent differences in the scaling exponents (Hiroi and Monsi 1966; Dunn and 8 Sharitz 1990; Morris and Myerscough 1991; Morris 1996). To explore the effects of 9 fertility levels on the scaling exponent of M-N relationship, Morris (1996) had shown 10 evidence that λ increased with decreasing fertility, and was positive under the lowest 11 site (Fig. 2A). Zhen et al. (1997) investigated the scaling exponent between average 12 aboveground biomass and density of Picea mongolica in Tengger Desert, and 13 indicated that γ increased as the soil water content declined (Fig. 2B). Finally, our 14 data showed that positive scaling exponents were yielded under harsh environment, 15 and decreased with the improvement of abiotic conditions (Fig. 1). Considered 16 together, these analysis demonstrated strong shifts from negative M-N relationship in 17 benign environments (-1/2 or -1/3) to positive under extremely stressful environments, 18 and different M-N relationships reflected variations in plant interaction in stands along 19 environmental gradients within the one species (Morris 1999). Hence, EER was not 20 applicable to all species under the all growth conditions. 21 The positive scaling might be attributed to the improvement of spatial utility 22 efficiency caused by the positive interactions. In that case, the size of an individual 23 would increase with density because of the positive effect of the neighboring plants. 24 Our study contributed to understanding the M-N relationships under harsh 25 environments. However, it was far from providing a real explanation, and new and 26 adequate approaches should be developed to investigate M-N relationship under 27 different resource-limited or stress conditions. 28 Materials and methods 29 30 The Gmelin larch (Larix gmelinii (Rupr.)) forest ecosystems located in the northern 9 1 half of Krasnoyarsk Region in Central Siberia (64o19′-71oN, 100o13′E) with 200 m 2 elevation and about 322 mm annual mean precipitation, which is the only forest 3 biome in the world where the continuous permafrost lies across most of their 4 distribution ranges (Bondarev 1997). Ecosystem type was larch forest ecosystems on 5 continuous permafrost, and characteristics of the forests in these regions were 6 described in detail elsewhere (e.g. Bondarev 1997; Osawa et al. 2000). Combined 7 effect of nitrogen limitation and low soil temperature results in an extremely stressful 8 region for forest (Osawa et al. 2003). 9 The second experiment site located in southeast of Minqin County (39o06′N, 10 104o02′E, 1707 m elevation), on the eastern edge of oasis-desert ecotone of Minqin, 11 northwestern of China, which is adjacent to the Tengger Desert with mobile, 12 semi-mobile, and static dunes. The study area is characterized by the arid continental 13 monsoon climate with strong northwest wind from Hexi Corridor in winter and spring 14 (Xu 1995). Average annual temperature is 7.6 ℃. Mean precipitation is 110 mm, with 15 most of it occurring from July to September while estimated potential 16 evapotranspiration is about 2 664 mm. The annual evaporation is nearly 24 folds of 17 the annual precipitation. The mineralization degree of water quality is 8-10 g· L - 1 and 18 groundwater is buried at 17 m underground ((Ma et al. 2003). In recent years, Minqin 19 has been threatened by desertification and became a typical location with shrinkage of 20 vegetation along the desert fringes of China (Ma et al. 2003). As affected 21 continuously by the evident descent of the underground water table and the rise of the 22 soil saline degree for past 40 years, a great multitude of seeds of wood and shrub 23 plants could not survive for renewal, especially the number of A. arenaria declined 24 obviously, while a xerphilization shrub plant Nitraria tungutorun has progressively 25 emerged, which will develop into their prosperity period and form climax desert plant 26 species under trend of succession (Yang 1995, 1999). At present environmental 27 deterioration has led to an extremely stressful region for growth and reproduction of 28 desert shrub A. arenaria. 29 The data of L. gmelinii from 118 grime in larch stands were analyzed using the 30 published data of Bondarev (1997) (40 stands) and Usoltsev (2001) (78 stands). These 10 1 data were divided into three categories: site indices I-IV presented relatively better 2 conditions, site index V was somewhat worse than in indices of I-IV, and Va-Vb 3 presented the poorest site condition, respectively (Usoltsev 2002, see Fig 1). The data 4 of A. arenaria were collected in July 2004 at Minqin site. The quadrates of 4m×4m 5 were randomly selected in 34 even-aged pure stands across the study area. In each 6 sample quadrates, the number of individuals (density) was measured, and the 7 aboveground biomass was harvested at ground level, then oven-dried at 80℃ for 48 h, 8 and weighed. 9 The M-N relationship was evaluated by the reduced major axis (RMA) regression 10 of log-transformed data, using RMA 1.17 (Hamilton et al. 2004; http://www.bio.sdsu. 11 edu/pub/andy/RMAmanual.pdf). 12 13 Acknowledgements 14 15 We sincerely appreciated A. Osawa for providing helpful information,and L. 16 Niklas and Yan-Jiang Luo for making comments to this paper. We also thank the two 17 anonymous reviewers for helpful comments on the manuscript. We acknowledge the 18 support of National Natural Science Foundation of China (90102015, 30170161). 19 20 References 21 22 23 24 25 26 1. Armas C, Ordials R, Pugnaire FI (2004). Measuring plant interactions: A new comparative index. Ecology 85, 2682-2686. 2. Bertness MD, Callaway RM (1994). Positive interaction in communities. Trends Ecol Evol 9, 191-193. 3. Bondarev A (1997). Aged distribution patterns in open boreal Dahurian larch 11 1 2 3 4 5 forests of Central Siberial. For Ecol Manage 93, 205-214. 4. Brown JH (1995). Macroecology. University of Chicago Press, Chicago, Illinois, USA. 5. Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004). Toward a metabolic theory of ecology. Ecology 85, 1771-1789. 6 6. Bruno JF, Bertness MD (2001). Habitat modification and facilitation in benthic 7 marine communities. In: Bertness MD, Hay ME, Gaines SD, eds. Marine 8 Community Ecology. Sinauer, Sunderland, MA. pp. 201-218. 9 10 7. Bruno JF, Stachowicz JJ, Bertness MD (2003). Inclusion of facilitation into ecological theory. Trends Ecol Evol 18, 119-125. 11 8. Callaway RM (1995). Positive interactions among plants. Bot Rev 61, 306-349. 12 9. Callaway RM, Walker LR (1997). Competition and facilitation: a synthetic 13 14 15 16 17 18 19 approach to interactions in plant communities. Ecology 78, 1958-1965. 10. Callaway RM, Brooker RW, Choler P, Kikvidze Z et al. (2002). Positive interactions among alpine plants increase with stress. Nature 417, 844-848. 11. Carbone C, Gittleman JL (2002). A Common Rule for the Scaling of Carnivore Density. Science 295, 2273-2276. 12. Cheng DL, Niklas KJ (2007). Above- and Below-ground Biomass Relationships Across 1534 Forested Communities. Ann Bot 99, 95-102. 20 13. Damuth JD (1998). Common rules for animals and plants. Nature 395, 115-116. 21 14. Dewar RC (1993). Mechanistic analysis of self-thinning in term of the carbon 22 balance of trees. Ann Bot 71, 147-159. 12 1 15. Dunn CP, Sharitz RR (1990). The relationship of light and plant geometry to 2 self-thinning of an aquatic annual herb. Murdannia keisak New phytol 115, 3 559-565. 4 5 6 7 16. Enquist BJ, Niklas KJ (2001). Invariant scaling relations across tree dominated communities. Nature 410, 655-660. 17. Enquist BJ, Brown JH, West GB (1998). Allometric scaling of plant energetics and population density. Nature 395, 163-165. 8 18. Enquist BJ, West GB, Charnov EL, Brown JH (1999). Allometric scaling of 9 production and life history variation in vascular plants. Nature 401, 907-911. 10 19. Ford ED (1975). Competition and stand structure in some even-aged plant 11 monocultures. J Ecol 63, 311-333. 12 20. Grime JP (1979). Plant Strategies and Vegetation Processes. Wiley, New York. 13 21. Hagihara A (1999). Theorical considerations on the C-D effect in self thinning 14 15 16 plant population. Res Popul Ecol 41, 151-159. 22. Harper JL (1977). Population Biology of Plants. London. Academic Press. pp. 25-31. 17 23. Hamilton AJ, Schellhorn NA Endersby NM Ridland PM, Ward SA (2004). A 18 dynamic binomial sequential sampling plan for Plutella xylostella (Lepidoptera : 19 Plutellidae) on broccoli and cauliflower in Australia. J Econ Entomol 97, 20 127-135. 21 24. Hamilton NRS, Matthew C, Lenaire G (1995). In defence of the -2/3 boundary 22 rule: a re-evaluation of self-thinning concepts and status. Ann Bot 76, 567-577. 13 1 25. Hector A, Schmid B, Beierkuhnlein C, Caldeira MC, Diemer M, 2 Dimitrakopoulos PG et al. (1999). Plant diversity and productivity experiments 3 in European grasslands. Science 286, 1123-1127. 4 26. Hiroi T, Monsi M (1966). Dry-matter economy of Helianthus annulus 5 communities grown at varying densities and light intensities. J Fac Sci Univ 6 Tokyo 9, 241-285. 7 8 9 10 27. Hutchings MJ, Budd CS (1981) Plant competition and its course through time. Bioscience 31, 640-645. 28. Hutchings, MJ (1983). Ecology’s law in search of the theory. New Scientist 98, 765-767. 11 29. Kira TH, Ogawa H, Sakazaki N (1953). Intraspecific competition among higher 12 plants. I. Competition-yield density interrelationship in regularly dispersed 13 populations. J Biol Osaka City Univ 4, 1-16. 14 15 16 17 30. Lonsdale WM (1990). The self-thinning rule: dead or alive? Ecology 71, 1373-1388. 31. Li HT, Han XG, Wu JG (2006). Variant scaling relationship for mass-density across tree-dominated communities. J Integrat Plant Biol 48, 268-277. 18 32. Ma XW, Li BG, Wu CR, Peng HJ, Guo YZ (2003). Predicting of temporal- 19 spatial change of groundwater table resulted from current land- use in Minqin 20 oasis. Adv Water Sci 14, 85-90 (in Chinese with an English abstract). 21 22 33. Morris EC (1999). Density-dependent mortality induced by low nutrient status of the substrate. Ann Bot 84, 95-107. 14 1 2 3 4 34. Morris EC (2003). How does fertility of the substrate affect intraspecific competition? Evidence and synthesis from self-thinning. Ecol Res 18, 287-305. 35. Morris EC, Myerscough PJ (1991). Self-thinning and competition intensity over a gradient of nutrient. J Ecol 79, 903-923. 5 36. Niklas KJ, Midgley JJ, Enquist BJ (2003). A general model for 6 mass-growth-density relations across tree-dominated communities. Evol Ecol Res 7 5, 1608-1617. 8 37. Osawa A (1995). Inverse relationship of crown fractural dimension to 9 self-thinning exponent of tree populations: a hypothesis. Can J For Res 25, 10 11 12 1608-1617. 38. Osawa A, Allen RB (1993). Allometric theory explains self-thinning relationships of mountain beech and red pine. Ecology 74, 1020-1032. 13 39. Osawa A, Abaimov AP, Zyryanova OA (2000). Reconstructing structural 14 development of even-aged larch stands in Siberia. Can J For Res 30, 580-588. 15 40. Osawa A, Abaimov AP, Matsuura Y, Kajimoto T, Zyryanova OA (2003). 16 Anomalous patterns of stand development in larch forests of Siberia. Tohku 17 Geophys J 36, 471-474. 18 41. Scrosati R (2005). Review of studies on biomass-density relationships (including 19 self-thinninglines) in seaweeds: main contributions and persisting misconceptions. 20 Phycol Res 53:224–33. 21 22 42. Stoll P, Prati D (2001). Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82, 319-327. 15 1 2 3 4 5 6 43. Tewksbury JJ, Lloyd JD (2001). Positive interactions under nurse-plants: spatial scale, stress gradients and benefactor size. Oecologia 127, 425-434. 44. Tilman D (1988). Plants Strategies and the Dynamics and Structure of Plant Communities. Princeton University Press, Princeton, NJ. 45. Tirado R, Pugnaire FR (2003). Shrub spatial aggregation and consequences for reproductive success. Oecologia 136, 296-301. 7 46. Usoltsev VA (2001). Forest biomass of northern Eurasia: date base and 8 geography. Ural Branch of Russian Academy of science, Yekaterinburg (in 9 Russian). 10 47. Usoltsev VA (2002). Forest biomass of northern Eurasia: mensuration standards 11 and geography. Ural Branch of Russian Academy of science, Yekaterinburg (in 12 Russian). 13 48. Weller DE (1989). The interspecific size-density relationship among crowded 14 plant stands and its implications for the –3/2 power rule of self-thinning. Am Nat 15 133, 20- 41. 16 17 49. West GB, Brown JH, Enquist BJ (1999). The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284, 1677-1679. 18 50. Westoby W (1984). The self-thinning rule. Adv Ecol Res 14, 167-226. 19 51. White J (1980). Demographic factors in population of plants. In O.T. Solbrig (ed.), 20 Demographic and evolution in plant populations. Berkeley, California: 21 University of California Press. pp. 21-48. 22 52. White J (1981). The allometric interpretation of self-thinning rule. J Theor Bio 16 1 89: 475-500. 2 53. White J (1985). The thinning rule and its application to mixture of plant 3 populations. In: White J, ed. Studies in plant demographic. New York: academic 4 press. pp. 291-309. 5 6 54. White J, Harper JL (1970). Correlated changes in plant size and number in plant populations. J Ecol 85, 467-485. 7 55. Whitford WG (2002). Ecology of desert systems. Academic Press, London. 8 56. Whittaker RJ (1999). Scaling, energetics and diversity. Nature 401, 865-866. 9 57. Wilson JB, Agnew AD (1992). Positive-feedback switches in plant communities. 10 11 12 13 14 Adv Ecol Res 23, 263-336. 58. Xu Y (1995). The climate characteristics of Minqin desert area. J Gansu For Sci Tech 3, 29-33 (in Chinese with English abstract). 59. Yang Z (1995). A preliminary study on the desert vegetation in Minqin. J Gansu For Sci Tech 3, 26-29 (in Chinese with English abstract). 15 60. Yang Z (1999). Research on desert vegetation changes for 40 years at shajingzi 16 area in Minqin. J Desert Res 19, 395-399 (in Chinese with English abstract). 17 61. Yastrebov, AB (1996). Different types of heterogeneity and plant competition in 18 monospecific stands. Oikos 75, 89–97. 19 62. Yoda K, Kira T, Ogawa H, Hozumi K (1963). Self-thinning in overcrowded 20 pure stands under cultivated and natural conditions (Intraspecific competition 21 among higher plants XI). J Biol Osaka City Univ 14, 107-129. 22 63. Zeide B (1987). Analysis of the 3/2 power law of self-thinning. For Sci 33, 17 1 517-537. 2 64. Zhen YR, Zhang XS, Xu WZ (1997). Regulative law of spruce population on 3 sand land. Acta Phytoecologica Sinica 21, 312-318 (in Chinese with English 4 abstract). 5 18 1 Figure legends: 2 3 4 5 6 7 Figure 1 Relationship between aboveground biomass and population density for two natural populations (data from Morris 1996). (1A) Larix gmelinii. The regression equations shown are log M = 4.36+1.19 log N in Va-Vb (●). The scaling exponent in I-IV(△) and V (×) were zero (P < 0.0001; R2=0.51). (1B) Artemisia arenaria. The regression equation shown is log M = 2.30 + 1.87 log N by RMA regressions of log-transformed data (P < 0.0001; R2=0.60). 8 9 10 11 12 13 14 15 16 Figure 2 Relationships between regression slopes for the aboveground biomass with density and abiotic conditions (data from Zhen et al. 1997). (2A) Ocimum basilicum. Three fertility levels significantly influenced the scaling exponents: λ increased as the fertility levels declined. The scaling exponent of aboveground biomass and density was -0.5, 0 and 0.94 at F2, F1 and F0 fertility level, respectively. (Morris 1996). (2B) Picea mongolica. The scaling exponent of average aboveground biomass and density increased as soil water content declined in Tengger Desert. γ was -0.91, -1.16 and -1.30 at 5.1%, 17.1% and 31.5% soil water content, respectively. 17 18 19 20 21 22 19 10000 2 Aboveground biomass (g/m ) 1A 1000 100 Site index I-IV Site index V Site index Va-Vb 10 1E-3 0.01 0.1 1 2 Stand density (no./m ) 1 1B 2 Aboveground biomass (g/m ) 100 10 1 0.1 0.1 1 2 Stand density (no./m ) 2 3 4 5 6 7 8 Figure 1 Relationship between aboveground biomass and population density for two natural populations (data from Morris 1996). (1A) Larix gmelinii. The regression equations shown are log M = 4.36+1.19 log N in Va-Vb (●). The scaling exponent in I-IV(△) and V (×) were zero (P < 0.0001; R2=0.51). (1B) Artemisia arenaria. The regression equation shown is log M = 2.30 + 1.87 log N by RMA regressions of log-transformed data (P < 0.0001; R2=0.60). 9 10 11 20 1.2 2A 1.0 Scaling Exponent (M-N) 0.8 0.6 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 F0 F1 F2 Fertility Levels 1 -0.90 2B -0.95 Scaling Exponent (m-N) -1.00 -1.05 -1.10 -1.15 -1.20 -1.25 -1.30 -1.35 -1.40 0 5 10 15 20 25 30 35 Soil Water Content (%) 2 3 4 5 6 7 8 9 10 Figure 2 Relationships between regression slopes for the aboveground biomass with density and abiotic conditions (data from Zhen et al. 1997). (2A) Ocimum basilicum. Three fertility levels significantly influenced the scaling exponents: λ increased as the fertility levels declined. The scaling exponent of aboveground biomass and density was -0.5, 0 and 0.94 at F2, F1 and F0 fertility level, respectively. (Morris 1996). (2B) Picea mongolica. The scaling exponent of average aboveground biomass and density increased as soil water content declined in Tengger Desert. γ was -0.91, -1.16 and -1.30 at 5.1%, 17.1% and 31.5% soil water content, respectively. 11 21