BcL-2 Family of Proteins and Role of Mitochondria in Cell Death
advertisement

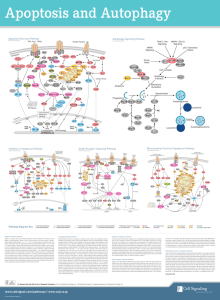
Mitochondria and Cell death: In addition to generation ATP and NADH (citric acid cycle) mitochondria have been shown to play critical role in cell death process. Disruption of membrane potential across inner mitochondrial membrane is observed very early during apoptosis after induction of cell death. Recently it has been established that release of cytochrome C, apoptosis initiating factor (AIF) from mitochondria leads to the activation of effecter caspases which are involved in execution of apoptosis. Since mitochondria are the main site for oxidative phosphorylation and utilization of molecular oxygen, they become the site for the generation of ROS if dysfunctional. The mechanisms of release of AIF, Cyt-c and caspase-9 from mitochondria during apoptosis is still not under stood. It has been observed that the permeability transition pores (PTP) open during apoptosis. PTPs are megachannel assemblies, present at the site where inner and outer mitochondrial membranes join together. Using immuno-precipitation analysis, it has been establish that following proteins may be involved in the frmation of PTPs. Voltage dependent anion channel (VDAC), adenine nucleotide transporter (ANT), Haxose kinase, peripheral benzodiazepine receptors (pBzR). Resently it was observed that the proapoptotic protein Bax associates with ANT and VDAC, indicating its role in PTP opening. The anti apoptotic protein Bcl-2 has been shown to bind to pBzR. This binding may keep the PTPs from opening and thus protect the cells from dying. The Ca ions and ROS may also affect the opening of PTPs as shown in the picture C. Opening of PTPs may cause release of only small molecules like ions, sugars, nucleotides. What is the mechanism of release of apoptotic proteins remains to be answered. BcL-2 Family of Proteins and Role of Mitochondria in Cell Death Bcl-2 proto-oncogene was discovered as a result of it involvement in lymphoma where it was translocated from chromosome 18 band q21 to Chromosome 14 q32 locus where it is in cloe vicinity of IgH gene. This brings an anti-apoptotic gene under the controle of strong enhancer element resulting in overproduction of Bcl2-2 protein. Bcl-2 is the most potent physiological inhibitor of apoptosis. Later on many homologues of Bcl-2 protein were discovered. Common feature of members of Bcl-2 family is the presence of Bcl-2 homology regions (BH1-BH4). These domains determine the interactions among different Bcl-2 proteins, interactions with other death related proteins (Bag and Raf-1) as well as their integration in the membranes. See the table for details of different members of this family. There are two categories of these proteins; Pro-apoptotic and anti-apoptotic Bcl-2 family of proteins. The trans membrane domain of Bcl-2 and Bax are essential for their function as anti-apoptotic and pro-apoptotic functions respectively. NMR spectroscopy and X-ray crystallography studies have indicated that similarity of Bcl-xL protein to pore forming colicins and deptheria toxins. Bcl-2 and Bax proteins have been shown to form ion channels in artificial membranes. Bcl-2 forms channels selective to K+ ions Bax forms channels selective to Cl- ions Regulation of Bcl-2 family of proteins by post-translational modifications: Phosphorylation: Bcl-2 is found to be phosphorylated in vivo and phosphorylated protein may have more of less antiapoptotic activity depending on the cell type, and nature of apoptotic stimulus and differences in phosphorylation sites. BAD interacts with BclxL at membrane sites only in un-phosphorylated form. Phosphorylation of BAD leads to dissociation of antiapoptotic BclxL which binds to Bax and stops apopsis. Bax has also shown to be phosphorylated by serine threonine kinases in vitro and may be regulated by phosphorylation. Proteolysis: So far only BID protein has been shown to be cleaved by caspase-8, which leads to its binding to Bax and mitochondrial membrane permeabilization. Mechanism of Action: Over-expression of Bcl-2 in cells has been shown to have inhibitory effect on apoptosis; 1. Prevents phoshptidylserine exposure at the cell surface 2. Inhibition of ROS generation and preventation of lipid peroxidation. 3. Inhibits activation of caspases 3 and 6. 4. Inhibits outflow of Ca from mitochondria and ER, and inflow into nucleus. 5. Inhibits pre-apoptotic disruption of mitochondrial membrane potential. 6. Inhibits PTP opening and release of AIF, cytochrome C from mitochondrial intermembrane space into cytoplasm. Based on these observations, one can predict multiple biochemical activities, following are some of the findings which throw some light on the mechanism of action of the Bcl-2 family member proteins In general cells life and death is decided by the ratio of anti-apoptotic and proapoptotic Bcl-2 related proteins. Death stimulus: lead to increase in the Bax/Bcl-2 ratio Survival signals: lead to decrease in Bax/Bcl-2 ratio Example: mammary glands epithelial cells: EGF and prolactin increase Bcl-2 and decrease Bax expression leading to proliferation and growth of these cells On the other hand treatment with TGF- beta 1 results in the increased expression of Bax. The Bax and Bcl-xs levels increase in mammary epithelial cells within first day after weaning. Physical interactions: Anti-apoptotic Bcl-2 family of proteins: Main site of action of Bcl-2 family of proteins is outer mitochondrial membrane. Bcl-2 heterodimerizes with Bax and prevents incorporation of Bax in to Permeability transition pores (PTP). Bcl-2 related anti-apoptotic proteins form complexes with APAF-1 (Ced-4) and some procaspases thereby they may inhibit activation of caspases. Proapoptotic Bcl-2 family of proteins may destabilize these complexes and release APAF-1 and activation of procaspases. Increased Bcl-2 level has been shown to protect cells from oxidative stress mediated cell death. Bcl-2 may regulate the flux of Ca across the mitochondrial, ER and nuclear membranes which is involved in the regulation of ROS production. Pro-apoptotic Bcl-2 family members: Pro-apoptotic protein Bax protein associates with voltage dependent anion channel protein (VDAC) and Adenine nucleotide tralslocase (ANT) at the contact site between outer and inner mitochondrial membranes and it may be involved in opening of the PTPs and release of AIF, Cytochrome C and Ca ions from mitochondria. Pro-apoptotic BID protein, after activation by proteolytic cleavage by caspase-8, associates with BAX and enhances its activity to integrate in mitochondrial membrane and cause release of AIF, cyt.C . Open questions: How Bcl-2 affects Ca ion flux through membranes of mitochondria, ER and nuclear membranes How Bax association with mitochondria causes release of AIF and cyt-c. Can Bcl-2 directly inhibit caspases or other proteases. Reference: Tatton and Onlaw (1999) BBA 1410: 195-213. Crompton, M (2000) Current Opinion in Cell Biol. 12: 414-419. Strasser, A, O’Connor, L and Dixit, V. M. (2000) Annual Rev. Biochem. 69: 217245. Motyl Tomasz (1999) Reprod. Nutr. Develop. 39: 49-59.