Class:
advertisement

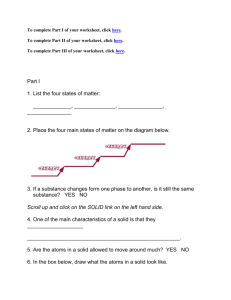
STATES OF MATTER Class: Scientific Principles Instructor: Abigail Carter Perryville High School Subject Area: Ninth Grade General Science Chemistry Unit Curriculum Objective: The students will be able to identify the four states of matter found on Earth and explain energy transfer between these four. (This lesson will take two 50-minute periods.) Technology Objective: Content Standard Alignment: (Missouri Show-Me Standards) Sc-1, Sc-5, Sc-8 Process Standard Alignment: (Missouri Show-Me Standards) Goal 1-8, 1-10, Goal 2-1 Learner Activity: Demonstration, kinesthetic activity, organizing information into a diagram, graphing skills Assessment Activity: Students will prepare a diagram of the four states of matter and the energy transfer between them. Method of Assessment: In their diagram, students should show an increasing amount of energy needed to change matter from solid, liquid, gas, and plasma. Instructional Method: DAY 1 Bellwork: Demonstration is set up at the front of the room. A beaker of ice, a beaker of water, and a beaker of water boiling on the hot plate (in that order). Students take vocab notecards – solid, liquid, and gases – and label what they see up front. Purpose: (written on the board) How do we change from one state of matter into another? Modeling/Guided Practice: Instructor takes the rest of the cards (except for plasma) and asks the students for help to label the demonstration. Solid Melt Freeze Evaporation liquid gas Condensation Sublimation Guided Practice: notes on vocabulary discussed above – 3 states of matter on Earth. What is actually happening during evaporation or condensation? Notes on increasing or decreasing amounts of energy. Kinesthetic Activity: Look at H2O at the particle level. Pass 2-3 marbles (H2O particles) out to each student. Students hold the particles in two hands. Model the movement of solid particles (move only slightly). Model the movement of liquid particles (move faster). Model the movement of solid particles (shake very fast – cup hands closed). Go back and forth and discuss what you must do with your hands in order the make the particles move faster/slower. Check for Understanding/Closure: Go back to the beaker demonstration. Student volunteers use the arrow note cards to show increasing or decreasing energy between states of matter. DAY 2 Bellwork: Review your notes from yesterday. I lied to you about something yesterday – can anyone figure out what I lied about. Purpose: Explain to the students that yesterday we discussed 3 states of matter on Earth – when there are actually 4. We will learn about the fourth today – although it is not very common on Earth. Guided Practice: notes on plasma – definition and where it can be found. Independent Practice: Reading on the use of and the principle behind plasma cutters (copied from a welding textbook or information found on the internet). Guided Practice: discuss reading and how a plasma cutter works. Do you increase or decrease energy when changing from a gas to a plasma state? Independent Practice: Create (or fill in on a worksheet) and diagram of energy changes between the four states of matter. Use all of the vocabulary words from these last two days. Think of the demonstration we used yesterday. Independent Practice (accelerated): Create a graph of energy changes between the four states of matter. Use all of the vocabulary words from these last two days. Think of the demonstration we used yesterday. Energy in the independent variable, states of matter are the dependent variables. Check for Understanding/Closure: Volunteer student recreates his/her diagram/graph on the overhead and explains. Resources: Vocabulary Word Note cards – words written largely on individual large index cards (solid, liquid, gas, plasma, freeze, melt, evaporation, condensation, sublimation), large note cards with arrows drawn on them, 3 large beakers, one hot plate, ice, water, overhead projector