Solids in Liquids
advertisement

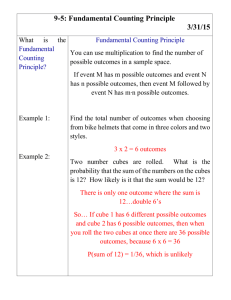
11-4 Sinking D2O Cubes, Floating Ice Cubes Description: Ice cubes and a cube of frozen deuterium oxide are dropped into beakers containing distilled water. After the hygroscopic D2O cube has lost its outer coating of H2O, it will sink. The regular ice cubes will remain floating on top of the liquid H2O. Cubes of benzyl alcohol and cyclohexane can also be created to demonstrate that other substances are typically more dense in their solid form than they are in their liquid form. Concept: When water freezes the structure expands so that the solid water is less dense than the liquid form. This is not the case for mostly all other substances. Deuterium oxide should sink in a container of water, since it contains a heavier isotope of Hydrogen. Materials: Deuterium oxide Distilled water Freezer for storage and creating D2O cube Deuterium oxide degassing/freezing chamber (see diagram on next page) 2-4, 600mL or 800mL beakers 2 ice and rock salt baths in ice buckets (for ice cubes and D2O cube) Ice bucket for chilling tongs 2-4 pairs of tongs Heat/cold insulating gloves Vacuum pump Aluminum foil (for covering dewars containing acetone baths) Vizcam 2000 (camera to project the image onto the large screen in rm 122) Optional: Benzyl alcohol Cylcohexane Cyclohexane degassing/freezing chamber (see diagram on next page) 2 acetone and dry ice baths in dewars (for cyclohexane and benzyl alcohol cubes only) 50mL beaker Dewar full of liquid nitrogen 2 tall-form 300mL beakers with solid #13 stoppers (for storing cubes in dry ice baths) 11-4 Diagram of the D2O Degassing/Freezing Chamber Diagram of the Cyclohexane Degassing/Freezing Chamber 11-4 Procedure: Creating Ice Cubes: Since the desired effect is to have a floating ice cube, the removal of trapped gases is not necessary. These cubes can be created by simply filling an ice cube tray with distilled water and storing it in the freezer. Before the lecture, free a few cubes from the tray and store them in a beaker that is submerged in an rock salt/ice bath. Creating a D2O Cube: Gases trapped within a D2O cube will lower the density and result in an undesirable, floating cube. By using the freezing chamber described above, most gases can be removed from the D2O before freezing occurs. Deuterium oxide that has been recently removed from a refrigerator should be added to the ice cube tray section with a transfer pipet once the section has been placed firmly in the sand. Chilling the D2O ahead of time prevents the liquid from boiling under the reduced pressure created. Once the tray section is full and the stopper is on the chamber, the vacuum pump should be connected just long enough to collapse the rubber tubing. The pump can then be shut off. Before disconnecting the tubing from the pump, a pinch clamp should be used to stop air from entering the chamber. The whole chamber can then be placed into a freezer. Before the lecture, remove the D2O cube from the chamber and store it in a beaker that is submerged in a rock salt/ice bath. Creating a Benzyl Alcohol Cube: Fill a 50mL beaker about ½ way full of benzyl alcohol. Holding the rim of the beaker with tongs, lower it into a dry ice/acetone bath and allow the substance to slowly freeze. Be patient, as the center of the cube will not form right away. Once a solid cube has been created, warm the outside of the beaker with a gloved hand until the cube is able to slide out. It is also possible to make the cube using a dewar of liquid nitrogen, though a rapid warming of the beaker afterwards may result in the beaker shattering. If this method does not produce a sinking cube, use the method described for creating a cyclohexane cube. Once a successful, sinking cube has been created, store the cube in a stoppered, 300mL tall-form beaker that is half submerged in a dry ice / acetone bath. Creating a Cyclohexane Cube: The cyclohexane cube, like the D2O cube, will float if gases are not removed prior to freezing. Since cyclohexane is more volatile than D2O, there is a greater chance of having gases trapped within a solid cube. A special piece of glassware has been created for degassing and freezing the cyclohexane. Fill the removable bottom half of this chamber with about 2 inches of chilled liquid cyclohexane. Connect the top half of the chamber and open the valve at the top. Connect the glass arm to a vacuum pump and begin drawing air out of the chamber. Once the pump has stopped pulling air through (there will be a noticeable change in sound), close the valve at the top. Slowly submerge the tip of the chamber into a dewar of liquid nitrogen. Continually dip the chamber into the liquid nitrogen so that a slow freezing of the liquid is allowed to occur. Once the cyclohexane is completely frozen, remove it from the liquid nitrogen and draw the gases from the chamber once more using the vacuum pump, making sure to reseal the chamber afterwards. Thaw the solid by running tap water over the outside of the chamber. Start by running cold water and gradually increase the temperature so that a sudden change in temperature does not shatter the glass. Repeat the degassing/freezing/degassing/thawing procedure a total of 3 times. After the last freezing step, open the chamber and warm the outside just enough to free the solid from the bottom. Test the cube to see if it will sink in cold cyclohexane and store it in a stoppered, 300mL tall-form beaker that is half submerged in one of the dry ice / acetone baths. 11-4 Safety: Work in the hood with the benzyl alcohol, cyclohexane, and acetone. Wear gloves and goggles. Avoid contact with the above solutions. Avoid contact with the deuterium oxide. When degassing, beware of over vacuuming, as the chambers can implode. Note: use tongs that have been chilled in an ice bath to handle the frozen solid pieces of benzyl alcohol and cyclohexane. These substances will melt quickly. During Class: Using the cold tongs drop a piece of solid benzyl alcohol into the beaker of liquid benzyl alcohol. If you do not need to use the piece again you can just let the solid melt in the beaker of liquid (this will happen relatively quickly). If you do need to re-use the cube, quickly retrieve it and put it back into the beaker in the acetone-dry ice bath. Using the cold tongs drop a piece of solid cylcohexane into the beaker of the liquid cyclohexane. If you do not need to use the pieces again you can just let the solid melt in the beaker of liquid (this will also happen relatively quickly). If you do need to re-use the cube, quickly retrieve it and put it back into the beaker in the acetone-dry ice bath. Use the tongs or your hands to put a few H2O ice cubes into the beaker of liquid H2O. Use the tongs to put a D2O cube into the same beaker of H2O. It is okay to let the H2O cubes melt, but please retrieve and save the D2O cube (D2O is rather expensive and we have a limited supply that is readily available). Clean-Up: Save the D2O, cyclohexane, and benzyl alcohol in their liquid state. Dump the salt-ice bath down the drain. Dump the liquid H2O down the drain. Allow the acetone-dry ice bath set until it has evaporated. Notes: Substance Benzyl alcohol Cyclohexane H2O D2O Melting Point (C) -15.19 6.47 0 3.81 11-4