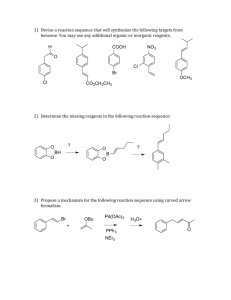
Chapter

18 th European Symposium on Computer Aided Process Engineering – ESCAPE 18
Bertrand Braunschweig and Xavier Joulia (Editors)
© 2008 Elsevier B.V./Ltd. All rights reserved.
OPTIMIZATION OF SYNTHESIS GAS
PRODUCTION PLANT USING RECYCLED
CARBON DIOXIDE
Seungjune Choi, Seunghyok Kim, Chonghun Han, En Sup Yoon
Department of chemical and biological engineering , Shillim -9dong, Kwanak-gu,
Seoul , Korea
Abstract
In this paper, the optimal design of multiple synthesis gas production process using recycled carbon dioxide is determined. The optimal configuration of the synthesis gas process is obtained by maximizing objective function based on the net of product revenue and manufacturing cost, and sustainable cost along with satisfying process constraints. We developed a superstructure of the global synthesis gas production process system which includes alternative synthesis gas reactions. The superstructure is the basis for the mixed integer linear programming that can be optimized to determine the best alternative reactions and operating conditions of the process. The industrial synthesis gas process problem is presented to illustrate the approach.
Keywords : Synthesis gas process, Superstructure, Optimization, Carbon dioxide recycle
1.
Introduction
Today energy consumption in modern societies is based on combustion of carbonaceous fuels, which are dominated by the fossil fuels: coal, petroleum, and natural gas.
Utilization of carbon dioxide has become a global issue due to the significant and continuous rise in atmospheric CO
2 concentrations which play an important role in the greenhouse effect, accelerated growth in the consumption of carbon-based energy worldwide, depletion of carbon-based fuel resources, and low efficiency in current energy systems.
Many articles were published that describe new methods and catalysts to utilize carbon dioxide for producing commercially important products. No single new technology will solve the entire problem of the carbon dioxide emission reductions. [Song (2002)] All of them have to overcome challenges of economics, performance, and associated environmental impacts which are some of the barriers identified to be able to make a new technology into widespread commercial use.
The one of the way to produce useful chemicals using CO
2 as a reactant or feedstock is the synthesis gas production. Synthesis gas is mixture of CO and H
2
and one of the most important feed stocks in the chemical industry. From synthesis gas mixtures having different H
2
/CO ratios a wide variety of products can be manufactured.
There are various methods for producing synthesis gas. Steam reforming of hydrocarbon is the most common method of production of synthesis gas. The other methods comprise dry methane reforming, partial oxidation of methane or reverse water gas shift reaction.
Though a lot of papers on production of synthesis gas using carbon dioxide were published, a few have focused on the optimization of the synthesis gas processes. From
2 S.Choi et al.
among these few papers on optimization, most of them are qualitative actually. They have tried to optimize variables or parameter of each synthesis gas production process; dry methane reforming, partial oxidation of methane or reverse water gas shift reaction process.
The objectives of this research are to identify and determine the optimal design of synthesis gas production processes that use carbon dioxide as a raw material. We developed a superstructure of the global synthesis gas production process system which includes alternative synthesis gas reactions. The optimal configuration of the synthesis gas process is obtained by maximizing objective function based on the net of product revenue and manufacturing cost.
2.
Synthesis gas production using carbon dioxide
2.1.
Steam reforming with recycled CO
2
Synthesis gas can be produced from a range of feed stocks (naphtha, coal, biomass etc.).
For heavy hydrocarbons as feedstock, partial oxidation with steam and oxygen is used for synthesis gas production. When producing synthesis gas production from natural gas or light hydrocarbons, steam reforming is the most widely used process if a high H
2
/CO ratio is desired. The process is typically operated at 15-30 bar and the outlet temperature of the reactor is typically 1123-1223K with a H
2
O/CH
4
ratio higher than 0.4.
CH + H O
4 2
CO + 3H (Steam reforming,+206 kJ/mol) (1)
2
CO + H O
CO + H (Water-gas-shift, WGS, -41 kJ/mol (2)
CH + CO
4 2
2CO + 2H
2
(Dry methane reforming, +247 kJ/mol) (3)
The steam reforming is highly endothermic and it is carried out at high temperature and at pressures between 15 and 30 bar. [Lu (1998)] The composition of the gas at the reactor outlet reflects the equilibrium of reaction (1) and (2) above.
In some cases, it is advantageous to add CO
2 at the inlet of the reformer. This is done in order to save hydrocarbon feedstock such as methane or LPG and decrease the H
2
/CO ratio in the product gas. The CO
2
coming out from the reformer is then recycled, and in some cases CO
2
is also imported. CO
2 then is supposed to react to form CO via the reverse WGS-reaction, and this is favored at low H
2
/CO ratios.
2.2.
Dry methane reforming (DMR)
The major source for synthesis gas is from the steam reforming reaction.
However, this process has several limitations like high energy requirement, a high
H
2
/CO product ratio, and poor selectivity for carbon monoxide. In addition, catalyst deactivation caused by carbon deposition is another problem.
An alternative to the steam reforming process is dry methane reforming (DMR).
This reaction has some advantages over steam reformer. It produces a synthesis gas with a lower H
2
/CO ratio and has higher energy efficiency in conversion to hydrocarbons. And it is effective way to utilize two greenhouse gases (CH
4
and CO
2
).
[Suhartanto (2001)]
In the DMR, steam is totally substituted by CO
2
, resulting in a H
2
/CO ratio of 0.42:1.
Because CO
2
reforming synthesis gas with a lower H
2
/CO ratio compared to steam reforming, this reaction can be used to adjust the H
2
/CO ratio in the product stream of steam reforming.
Optimization of Synthesis Gas Production Plant Using Recycled Carbon Dioxide
2.3.
Reverse water gas shift (RWGS) process
The water gas shift reaction has been intensively studied for the last several decades for
H2 production from synthesis gas. On the contrary, a reverse-water-gas-shift reaction has attracted little attention because of little demand. The water gas shift (WGS) Eq. (2), the reaction of carbon monoxide and water to produce carbon dioxide and hydrogen, is frequently used in industries in conjunction with the production of pure hydrogen via the steam reforming of hydrocarbons. [Joo (1999)] On the other hand, the reverse water gas shift (RWGS) Eq. (4), the reaction of carbon dioxide and hydrogen to produce carbon monoxide and water, is also a reaction of considerable industrial importance:
CO
2
+ H
2
→ CO + H
2
O (4)
3.
Superstructure optimization
3.1.
Generation of superstructure
The superstructure can identify its interconnections which have their corresponding feed compositions and flowrates that produce a maximization of total annualized profit of the synthesis gas production process. A superstructure for the synthesis gas production process, containing feasible alternative design is presented in a simplified form in Fig 1.
3
RWGS
Reactor 1
RWGS
Reactor 2
Fig 1. Superstructure of the process
In this superstructure, there are three types of commercially available synthesis gas production unit considered: RWGS, DMR, steam reforming with recycled carbon dioxide.
And in order to complete the superstructure, auxiliary units are included. So it consists of an existing steam reformer with recycled CO
2
, two DMR units, two RWGS units, a
MDEA unit for CO
2
separation, a membrane, a PSA (Pressure Swing Adsorption) unit, etc. The carbon dioxide from the each process is recycled from the MDEA process, CO
2 separator, to the feed stream. The separated synthesis gas is compressed and split into the carbon monoxide and hydrogen from the membrane and the hydrogen is refined in the PSA unit and partially recycled to the feed stream.
4 S.Choi et al.
3.2.
Mathematical formulation
Though the superstructure generated all feasible alternative of the synthesis gas process, it does not address process-specific description. This section presents the formulation of mathematical model of the synthesis process superstructure for the optimal process design. In order to develop mathematical model, continuous and binary variables are associated with the superstructure. The continuous variables represent the stream flowrates and capacities of the process units. And the binary variables assigned to the existence or the nonexistence of the corresponding process units at a given operating condition.
Mathematical expressions for constraints and cost functions are generated by the operating condition and environment of the process along with production capacities, product demand and raw material availability. And the annualized maximum profit of the process is also obtained from the objective function.
Hence, the proposed optimization model is formulated as shown below. a) Maximization of annualized process profit:
Max profit
PR
OC
RMC
CC
SC
PR
RMC
k
P
k
Prod
F
k
Prod
, OC
k k
P F
k
Feed Feed
, CC
(
i fix i
i ut
OC W OC G
i
W CC , SC
i i
),
tax
C ( F
CO
2 gen
F
CO
2 con
)
(5) b) Material balance
F
i in k
F
k i out
s
i k
d
i k
process unit
(6) c) Energy balance
Qs i
Qd i
H i k
(7)
H i k s i k
k
F i out
Cp k i out
Cp k s i
Cp k ref
2
k
Cp ref
2
( T i out
T ref
( T s i
T ref
)
F i in k
)
d i k
Cp k d i
Cp k ref
2
Cp k i in
Cp k ref
2
( T T d ref i
( T i in
T ref
)
)
(8) d) Conversion of key reactant and components
C
F i in k
(9) s i k d i k
(or )
i k i f) Variable bounds
0
i
UP
F F ; T i
LO
C (10)
i
UP
T T ; P
LO i
P
UP
,
i (11)
Optimization of Synthesis Gas Production Plant Using Recycled Carbon Dioxide
4.
Industrial case study
4.1.
Optimally designed synthesis gas production process with existing process
In this section, the industrial synthesis gas production plant with proposed carbon processes was optimized.
A carbon dioxide pipeline from the nearby plant can be connected to the conventional synthesis gas process of OCTANOL(2-Ethylhexanol) plant, and the cost of excess highpurity carbon dioxide is essentially pumping cost of about $3 per ton (2 metric ton per hour), since it is being vented to the atmosphere now. For saving the raw material cost and operation cost, the synthesis gas process was expanded into a superstructure by integrating the supplementary carbon dioxide processes. And they were set up to produce synthesis gas from the high purity carbon dioxide which is generated as a byproduct from the other adjacent plant.
The optimal configuration of the plant was obtained from the superstructure and it is shown in Fig. 2.
5
Fig 2. Optimal configuration of the plant. (Flow rates in metric ton per year)
The optimal structure included DMR process and direct feeding with recycled CO
2
for the synthesis gas production. It was more profitable to have the corresponding existing process present.
The net profit increased from $15.1 million per year to $17.5 million per year or more than 17% from the base case to the optimal structure. Although the product sales didn’t decrease much, the raw material and operating cost decreased from $24.3 million to
$21.2 million. Because it could cut down the raw material cost by using cheap carbon dioxide as raw material and operating cost by reducing the fuel for the steam reformer.
And sustainable cost also decreased about 75% from the base case as it consumed carbon dioxide.
However, the optimal structure could not consume all of the carbon dioxide available and 2.1 metric ton per hour, 69% of carbon dioxide brought from the other plant, is vented to atmosphere and it is imposed a carbon tax. So, the other extension to the optimization problem was evaluated.
4.2.
Maximizing CO
2
consumption
In this section, the optimal structure was determined maximizing CO
2
consumption.
This result has affected the optimal structure of process and it contained the RWGS process and DMR process for producing the synthesis gas (Fig 3).
6 S.Choi et al.
The net profit decreased further to $14.1 million per year having consumed about 2 metric ton of the CO
2
from the other plant. These declines are a result of changes in sales and all of the associated costs. In this case, the process could reduce sustainable cost by the decreased emission of carbon dioxide, but a decline of producing high pure hydrogen led to the diminution of total product sales and net profit. Because the RWGS process requires high pure hydrogen for the synthesis reaction, the productivity of hydrogen and total product sales decreased.
RWGS
Reactor 1
Fig 3. Maximizing CO
2
consumption process configuration
5.
Conclusion
In this paper, the optimal design of synthesis gas production process including multiple reaction paths has been identified. In order to determine the optimal design of synthesis gas production process utilizing various reaction paths using carbon dioxide, three kinds of alternative synthesis gas process are considered. We developed a superstructure of the global synthesis gas production process system which includes alternative synthesis gas reactions. Industrial studies are applied. And the optimal configurations of the synthesis gas processes have been obtained by maximizing objective function based on the net profit of product revenue and manufacturing and sustainable cost.
References
Joo, O., Jung K., Moon I., Ya, A., Galina, R., Lin, I., Han, S., Uhm, S., “Carbon Dioxide
Hydrogenation To Form Methanol via a Reverse-Water-Gas-Shift Reaction”, Ind. Eng. Chem.
Res., (1999) 38 (5), 1808 -1812
Lu, Y., Xue, J., Yu C., Liu Y., Shen, S., “Mechanistic investigations on the partial oxidation of methane to synthesis gas over a nickel-on-alumina catalyst”, Appl. Catal . A 174 (1998)
Song, C., Gaffney, A.F., Fujimoto, K. F., CO
2
conversion and utilization, (2002) ACS
Symposium Series 809, Chap 1. American Chemical Society Oxford University Press,
Washington
Suhartanto, T.; York, A. P. E.; Hanif, A.; Al-Megren, H.; Green, M. L. H. Potential utilization of
Indonesia's natural gas fields via methane dry reforming to synthesis gas. Catal. Lett., (2001)
21, 49.