Colloidal (Kol-oid-al) chemistry is not new, but it is not widely known
advertisement

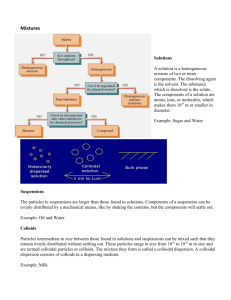
Colloidal (Kol-oid-al) chemistry is not new, but it is not widely known about or understood by the general public. Simply said, a colloid refers to a substance that exists as ultra-fine particles that are suspended in a medium of different matter. The colloidal state is the state of a solute (mineral or other substance such as a paint pigment) in a solution when its molecules do not separate into atoms as with a true solution (sodium chloride or salt separates into separate sodium and chloride atoms while in solution), but rather they remain grouped together to form solute particles. The presence of these inorganic colloidal particles, which are approximately one hundred-thousandth to one ten-millionth of a centimeter in diameter (about 400 thousandths to four millionths of an inch), can often be detected by means of an electron microscope. As a result of the grouping of the molecules, a solute in the colloidal state cannot pass through a suitable semipermeable membrane and gives rise to negligible osmotic pressure (they will pass through filter paper), depression of freezing point and elevation of boiling point effects. These ultra-fine particles of the colloid are just barely larger than most molecules and so small they can't be seen with the naked eye - about one billion of these colloid particles would fit into a cubic 0.01 of an inch. The "solution" part of a colloid provides a solid, gas or liquid medium in which the colloid particles are suspended. The suspended particles in a colloid can also be a solid, a gas or a liquid Solutions were classified by H Freundlich (1925) into three categories: True solutions Colloidal solutions Emulsions and suspensions. The four part method of classifying solutions is as follows: Identify particle size. Determine presence of Brownian movement (random movement of particles suspended in liquids or gasses resulting from the impact of molecules of the fluid surrounding the particles). Ability to pass through filter paper. Level of solubility In 1975, S. S. Voyutsky (a Russian) wrote the classic text on colloidal chemistry. Voyutsky referred to solutions as "molecular dispersion systems" and "heterogeneous highly dispersed colloidal systems." The exact point between the molecular and colloidal degrees of dispersion cannot be established because the transition from molecularly dispersed systems to coarsely dispersed systems is a continuous range. A colloidal system must have three basic characteristics: It must be heterogeneous (consists of dissimilar ingredients or constituents). The system must be multi-phasic (i.e.solid/liquid, gas/liquid, etc.). The particles must be insoluble (do not dissolve in the solution). Each one of these classifications interunique qualities. The interesting thing about colloids is that they remain heterogeneous, multi-phasic and insoluble at different concentrations as long as a larger number if not all of the particles are within the range of sizes of colloids ( In to 100n). The molecular groups or particles of the colloid solute carry a resultant electrical charge, generally of the same sign (negative) for all of the particles. A small percentage of these inorganic colloids will pass through the intestine of a living animal or human because a natural chelating process takes place in the gut in the presence of protein-containing food. Inorganic colloidal material which readily passes through filter paper may be separated from dissolved substances, such as starch, sugar or salt, by placing the mixture of mineral colloid and non colloid in a parchment shell surrounded by distilled water. The inorganic colloids are "too large" to pass through the membrane, but the molecules of salt, starch and sugar or any other dissolved substance pass readily through the semipermeable membrane (they separate into individual atoms or very small molecules). This kind of separation process is called dialysis. In the process of digestion the inorganic minerals in food or supplements soon become inorganic colloids and as an inorganic colloid they cannot penetrate the intestinal wall to enter the blood stream. In the presence of amino acids a small percentage of the inorganic colloids form chelated minerals and organic colloids which are able to be dialyzed through the mucus membranes of the intestinal walls into the blood stream - this form of bio available mineral state is known as a "crystalloid." Crystalloids or organic colloids readily pass through cell walls, while non-organic colloids are "too large." Additionally we must remember that in the living organism there are other physiological forces at work which interfere with or modify the expected osmotic phenomenon. Colloidal mineral supplements and commercial colloids are found in four different forms: Unprotected colloids are made of bare "rock flour," this is the form of inorganic metallic colloid found in sea bed minerals, clays, "soils," and "Glacial Milk." This form of inorganic colloid is in fact a metallic mineral and is only available to plants when there is a healthy soil population of bacteria and fungi. The second type of mineral colloid is found in the living systems of bacteria, fungi, green plants (food crops), animals and humans and is coated by a water loving (hydrophilic) substance such as gelatin, albumin, albuminoids, or collagen. This coating protects the now "organic mineral colloid" and allows it to be a crystalloid for absorption, storage and physiological uses and thus maximizing its bioavailabiIity to 98 %. The third type of organic mineral colloid has a protective coating of carbon with a molecular chain length of 10 to 12 carbon atoms. This type of colloid is also found in bacteria, fungi, plants (including some forms of petrified wood), animals and humans and is thought to be the most stable form of natural organic mineral colloid. The fourth type of mineral colloid is not found in nature, but rather is manufactured industrially by coating the metallic colloid with sulfated castor oil ( lipophillic or fat or oil loving) to form commercial detergents. Colloidal (Kol-oid-al) chemistry is not new, but it is not widely known about or understood by the general public. Simply said, a colloid refers to a substance that exists as ultra-fine particles that are suspended in a medium of different matter. The colloidal state is the state of a solute (mineral or other substance such as a paint pigment) in a solution when its molecules do not separate into atoms as with a true solution (sodium chloride or salt separates into separate sodium and chloride atoms while in solution), but rather they remain grouped together to form solute particles. The presence of these inorganic colloidal particles, which are approximately one hundred-thousandth to one ten-millionth of a centimeter in diameter (about 400 thousandths to four millionths of an inch), can often be detected by means of an electron microscope. As a result of the grouping of the molecules, a solute in the colloidal state cannot pass through a suitable semi permeable membrane and gives rise to negligible osmotic pressure (they will pass through filter paper), depression of freezing point and elevation of boiling point effects. These ultra-fine particles of the colloid are just barely larger than most molecules and so small they can't be seen with the naked eye - about one billion of these colloid particles would fit into a cubic 0.01 of an inch. The "solution" part of a colloid provides a solid, gas or liquid medium in which the colloid particles are suspended. The suspended particles in a colloid can also be a solid, a gas or a liquid Solutions were classified by H Freundlich (1925) into three categories: 1. True solutions 2. Colloidal solutions 3. Emulsions and suspensions. The four part method of classifying solutions is as follows: 1. Identify particle size. 2. Determine presence of Brownian movement (random movement of particles suspended in liquids or gasses resulting from the impact of molecules of the fluid surrounding the particles). 3. Ability to pass through filter paper. 4. Level of solubility In 1975, S. S. Voyutsky (a Russian) wrote the classic text on colloidal chemistry. Voyutsky referred to solutions as "molecular dispersion systems" and "heterogeneous highly dispersed colloidal systems." The exact point between the molecular and colloidal degrees of dispersion cannot be established because the transition from molecularly dispersed systems to coarsely dispersed systems is a continuous range. A colloidal system must have three basic characteristics: 1. It must be heterogeneous (consists of dissimilar ingredients or constituents). 2. The system must be multi-phasic (i.e. solid/liquid, gas/liquid, etc.). 3. The particles must be insoluble (do not dissolve in the solution). Each one of these classifications interunique qualities. The interesting thing about colloids is that they remain heterogeneous, multi-phasic and insoluble at different concentrations as long as a larger number if not all of the particles are within the range of sizes of colloids ( In to 100n). The molecular groups or particles of the colloid solute carry a resultant electrical charge, generally of the same sign (negative) for all of the particles. A small percentage of these inorganic colloids will pass through the intestine of a living animal or human because a natural chelating process takes place in the gut in the presence of protein-containing food. Inorganic colloidal material which readily passes through filter paper may be separated from dissolved substances, such as starch, sugar or salt, by placing the mixture of mineral colloid and non colloid in a parchment shell surrounded by distilled water. The inorganic colloids are "too large" to pass through the membrane, but the molecules of salt, starch and sugar or any other dissolved substance pass readily through the semi permeable membrane (they separate into individual atoms or very small molecules). This kind of separation process is called dialysis. In the process of digestion the inorganic minerals in food or supplements soon become inorganic colloids and as an inorganic colloid they cannot penetrate the intestinal wall to enter the blood stream. In the presence of amino acids a small percentage of the inorganic colloids form Chelated minerals and organic colloids which are able to be dialyzed through the mucus membranes of the intestinal walls into the blood stream - this form of bio available mineral state is known as a "crystalloid." Crystalloids or organic colloids readily pass through cell walls, while non-organic colloids are "too large." Additionally we must remember that in the living organism there are other physiological forces at work which interfere with or modify the expected osmotic phenomenon. Colloidal mineral supplements and commercial colloids are found in four different forms: 1. Unprotected colloids are made of bare "rock flour," this is the form of inorganic metallic colloid found in sea bed minerals, clays, "soils," and "Glacial Milk." This form of inorganic colloid is in fact a metallic mineral and is only available to plants when there is a healthy soil population of bacteria and fungi. 2. The second type of mineral colloid is found in the living systems of bacteria, fungi, green plants (food crops), animals and humans and is coated by a water loving (hydrophilic) substance such as gelatin, albumin, albuminoids, or collagen. This coating protects the now "organic mineral colloid" and allows it to be a crystalloid for absorption, storage and physiological uses and thus maximizing its bioavailability to 98 %. 3. The third type of organic mineral colloid has a protective coating of carbon with a molecular chain length of 10 to 12 carbon atoms. This type of colloid is also found in bacteria, fungi, plants (including some forms of petrified wood), animals and humans and is thought to be the most stable form of natural organic mineral colloid. 4. The fourth type of mineral colloid is not found in nature, but rather is manufactured industrially by coating the metallic colloid with sulfated castor oil ( lipophillic or fat or oil loving) to form commercial detergents.