Periplasmic protein expression – FimH truncate expression in E
advertisement

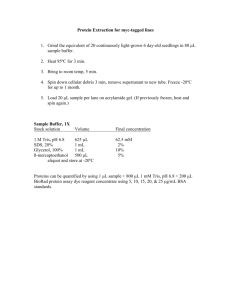
May 11th, Monday Periplasmic protein expression – FimH truncate expression in E. coli Sunday: Start overnight cultures of cells containing the FimH truncate expressionvector from glycerol stocks, (Gelareh) NB: Steps 1. and 2. have to be carried out under sterile conditions. There is a great risk of phage contamination in the lab, which would lead to cell death and NO RESULTS! So, please prepare everything with outmost care! Preparation of large cultures of FimHtr: 1. Inoculation: i) Prepare two flasks with a final volume of no more than 700 ml in each. In the following order: Add ampicillin to a final concentration of 100 mg/l to each flask, i.e. 700 ul of the prepared stock solution 100 mg/ml. Add 665 ml of the prepared with an available carbohydrate binding site for later binding studies.minimal media# and finally 35 ml of the overnight culture. Note: FimH shows strong binding affinity for mannose derivatives. The lectin picks up a butyl -D-mannoside from the Luria-Bertani (LB) medium during expression, which is why minimal media is used instead. This provides proteins # Minimal media: For a 700 ml culture, prepare the media in the following order: 35 ml 20xM9 salt, 400 ml sterile water, 20 ml 20% Glucose, 85 ul CaCl2, the remaining water (210 ml) and finally 700 ul MgSO4. ii) Grow the cultures in shakers at 37 C and follow the growth curve by measuring the optical density of both cultures at 600 nm every hour. 2. Induction: i) The cultures should be ready to induce in their log phase, approximately at OD600 of 0.6-0.8 units (compare with your own growth curves). Take 1 ml of your non induced cultures to eppendorf tubes and spin down at 8000 rpm in a table top centrifuge for 10 min. Discard the supernatant and store in cold room for further analysis. i) Induce with isopropyl -D-thiopgalactopyranoside (IPTG) to a final concentration of 5 mM (i.e 350 ul of the 1M IPTG stock). ii) Incubate further for up to 2.5 h. To make sure that the cultures are ok, check the growth progress by measuring OD600 every hour. 3. Harvesting: i) Weigh the empty centrifuge flasks with lids and note it down and the balance the flasks carefully after the cultures are added. Spin down the cells at 4500rpm (swing out rotor) for 20 min. ii) While waiting – Add Jododpax to the culture flasks. After the centrifugation, empty the supernatant into the flasks and fill them up with water. It is very important to kill off all bacteria and other possible contaminants as soon as possible, in order to prevent a contamination for everyone else in the lab. 4. Periplasmic preparation: i) Discard the supernatant and weigh your cells. ii) Resuspend cells thoroughly in ice-cold sucrose solution: 20 mM Tris-HCl pH 8.0, 25 % (w/v) sucrose and 5 mM EDTA. Take 4 ml of sucrose mix per gram cell. Transfer the samples to 50 ml Falcon tubes and incubate on ice for 15 min. Note: This step creates a concentration gradient across membranes. ii) Spin down the samples at 8500 xg (table top centrifuge, fixed angle rotor) for 20 min. Remove the supernatant (the sucrose fraction) and save it on ice. iii) Extract the periplasmic content by osmotic shock. Dissolve the pellet in 4-5 ml per gram cell of a mix of ice-cold 5 mM MgCl2 and half a tablet of protease inhibitor cocktail (Roche). Add 40 ul of the lysozyme stock [15 mg/ml] per gram cell and incubate on ice for 30 min. iv) Spin down the samples at 8500 xg (fixed angle rotor) for 20 min. Take 50 ul of the supernatant (periplasmic fraction) for further analysis. 5. Overnight dialysis for protein purification: Dialyze the supernatant against 20 mM sodium phosphate buffer pH 7.8, in cold room under slow continuous stirring, over night. 6. SDS-PAGE to analyze the expression: Prepare a 15 % polyacrylamide SDS gel, using the Bio Rad system. Prepare the sucrose, pellet and periplasmic fractions according to the following: add 6 ul of 4x SDS loading buffer to 18 ul of the sucrose and periplasmic fractions. Dissolve some of the pellet in 18 ul of water and add 6 ul of the loading buffer. Boil the samples and a low molecular weight marker for 10 min at 95 C. Add 5 % -mercaptoethanol to each and spin down. Mount the gel and add buffer. NB: Check for leakage! Load approximately 15 ul of each sample and run at 200 V for approximately 40 min. Stain in Commasie blue stain. From Wet Lab 1: SDS-PAGE Prepare the gels like this: 15% separating gel, pH 8.8 ml 88% Glycerol 0.525 1M Tris-HCl, pH 8.8 1.87 30% AA-bisAA (30:1) 2.5 10% SDS 0.05 10% PSA 0.05 TEMED 0.005 5 ml 4% stacking gel, pH 6.8 H2O 1M Tris-HCl, pH 6.8 30% AA-bisAA (30:1) 10% SDS 10% PSA TEMED ml 1.84 0.312 0.325 0.025 0.0125 0.005 2.5 ml Assemble the glass plates and spacers. Pour the separating gel to about 1 cm below the wells of the comb. Pour the stacking gel and insert the comb immediately. When the stacking gel has set, place in gel rig and immerse in buffer. Prepare the samples: Take 30 ul of each sample and mix with 10 ul 4x SDS loading buffer containing 0.1 M -mercaptoethanol (added fresh before use) Heated the mixture at 100C for 5 min. Load the samples into the wells ( no more than 15 ul). Be sure to use markers. Run with constant voltage (200V) for about 1 hr. Staining the gel with Coomassie Blue: Place the gel in a box, eg. An empty tip box. Add the fix solution (30% EtOH, 10% HAc) and heat it to near boiling in the microwave oven (10 – 20 s). Pour the fix solution back for reuse the fix solution. Add 50 ml Coomassie Blue solution and heat it to near boiling. Incubate 5 min at room temperature; shake it every now and then. Pour the Coomassie solution back into its Falcon tube for reuse (can be reused up to 10 times). Rinse the gel with some water and heat it to near boiling. Incubate 5 min or longer at room temperature, shake it every now and then. Change the water and heat and incubate again until the background is low enough. The gel is now ready for scanning! May 13th, Tuesday Protein purification by liquid chromatography, using a HiTrap chelating column. 1. Analysis: Look at your gels and determine the success-rate of yesterdays results. Determine whether you have protein only in the periplasmic fraction or in the sucrose fraction as well. If there is protein in the sucrose fraction start a dialysis of this fragment as well. Reuse the same membrane but prepare fresh buffer. 2. Pre purification preparations: i) Sample preparation: Continue with the periplasmic fraction. To remove any precipitated proteins, before purification, centrifuge the dialyzed periplasmic fraction in Falcon tubes at 8500 rpm (fixed angle rotor) for 20 min. Filter the dialyzed supernatant before loading the sample onto the column, using 0.45 um sterile filters. ii) Buffer preperation: Filter and degas all solutions. Buffer A: 20 mM sodium phosphate buffer pH 7.8 (equilibration, loading, washing) Buffer B: 20 mM sodium phosphate buffer pH 7.8, 500 mM imidazole (elution) iii) Column preparation: Strip the column with EDTA solution, 3 column volumes (CV). Wash with water, 5 CV. Load on Ni-chloride, 3 CV. Wash off excess/unbound nickel with water, 5 CV. Column information Name: HiTrap Chelating Column (GE) Technique: Complex binding of the C-terminally His-tagged FimHtr with Nickel ion coated sepharose column matrix Column bed volume: 5 ml Maximum sample volume: 12 mg pure His-tagged protein per ml/medium Recommended flow rate: 5 ml/ min Maximum pressure: 0.5 MPa iv) Column equilibration: Wash the system and pump A and B with buffers A and B, respectively. Wash column with 3 CV buffer A, 3 CV buffer B and finally 5 CV of buffer A again. 5. Protein purification – periplasmic fraction: After discussing the purification steps with your lab-partners and teacher and then start the purification (we will run this step in manual mode). Note: Make sure to keep an eye on the fractionation and to not pump any air into the ÄKTA systems and columns. 6. Overnight dialysis for further purification step: Collect the FimHtr fractions and dialyze against 50 mM sodium acetate pH 5.25 (buffer A, which is going be used in the ion exchange step on Wednesday) under continuous stirring, over night. Take off 50 ul of the sample for SDS-PAGE. (7. Protein purification – sucrose fraction: Follow steps 5. & 6. again, but remember to re equlibrate the column with buffer A before applying the sample.) May 14th, Wednesday Protein purification, using a MonoS ion exchange column. 1. Pre purification preparations: i) Centrifuge the dialyzed protein sample(s) in Falcon tubes at 8500 rpm for 20 min and filter the supernatant, using 0.2 um sterile filters. Measure the concentration of the protein before loading it onto the column, using the Nano-drop. Take 50 ul of the sample(s) for further analysis. ii) Buffer preparation: Buffer A: 50 mM sodium acetate pH 5.25 (equilibration, loading, washing) Buffer B: 50 mM sodium acetate pH 5.25, 500 mM NaCl (elution) iii) Column preparation and equilibration: Wash the system and pumps. Wash the column with buffer A and B and equilibrate with buffer A. Column information Name: MonoS column (GE) Technique: Cation exchanger, binding of the positively charged protein. Column bed volume: 8 ml Recommended flow rate: 2 ml/ min Maximum pressure: 5 MPa 5. Protein purification: After discussing the purification steps with your lab-partners and teacher and then start the purification (we will run this step in manual mode). Note: Make sure to keep an eye on the fractionation and to not pump any air into the ÄKTA systems and columns. 6. SDS-PAGE to analyze the expression: Same protocol as above with a 15 % SDS gel to check the quantity and the purity of the protein sample after the before and after each purification step. 7. Measure the final concentration of the protein and store on ice.