Supplemental Materials Methods Real
advertisement

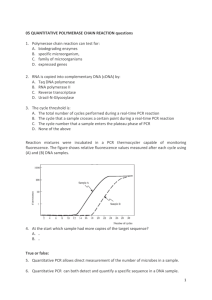
Supplemental Materials Methods Real-time quantitative PCR. DNA was extracted from samples by using the DNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Briefly, samples were incubated in 300 µl lysis buffer (see text) for 30 min at 37°C. Then 25 µl proteinase K and 200 µl buffer AL were added and incubated 30 min at 56 °C. Next, 200 µl AL buffer was added to the tube, the sample was vortexed, and 200 µl 100% ethanol was added. Finally, sample was loaded onto the silica-gel column, washed, and eluted in 100 µl elution buffer. To quantify the relative concentrations of mucosa-associated bifidobacteria, DNA samples were probed with primers designed to amplify segments of the 16s rRNA gene. The primers employed were as follows: Bif F (5’-TCGCGTCTGGTGTGAAAG-3’) and Bif R (5’-CCACATCCAGCGTCCAC3’) for B. infantis [59]. B. bifidum was analyzed using primers: BiBif F (5’-CCACATGATCGCATGTGATTG-3’) and BiBif R (5’-CCGAAGGCTTGCTCCCAAA-3’) [60]. A standard curve for quantification of Bifidobacteria strains was generated from serial dilutions of bacterial DNA and used to calculate numbers of bacterial copies. Estimates of the number of Bifidobacteria genome copies in the standard were based on a genome size (1.75 – 2.8 Mb) of the individual strain. DNA concentration was determined using NanoDrop (Wilmington, DE). Real-time PCR was performed in a 7500 Fast Real-Time PCR System (Applied Biosystems, CA) and SYBR Green fluorophore was used to correlate the amount of PCR product with the fluorescent signal. The PCR reactions and melting curves were performed in 20 µl which contained 1 µl of each primer, 10 µl SYBR Green PCR Master Mix 2x (Applied Biosystems, CA), and 2 µl of sample DNA. The PCR reaction was incubated at 95 °C for 10 min to activate the polymerase followed by 40 cycles consisting of denaturation for 20 s at 95 °C, annealing for 20 s 56 °C, and extension for 30 s at 60 °C. Fluorescence was monitored at the end of each extension phase. After amplification, melting curves were generated to verify PCR product identity. Adhesion of bacterial cells was shown as the percentage of the recovered cells vs. cells applied onto Caco-2 monolayers. Gene expression measurements. Real time reverse transcriptase (RT)-PCR was used to analyze cytokines and TJ mRNA levels in Caco-2 or HT-29 cells incubated with HMO-grown bifidobacteria. After 2h incubation total RNA was isolated from scrapings of epithelial cells using extraction kit (AllPrep DNA/RNA Mini Kit, Qiagen) (61). RNA was quantified using NanoDrop (Wilmington, DE). After reverse transcription reaction using Quantitect (Qiagen), real-time PCR quantitative mRNA analyses were performed using SYBR green fluorescence (Applied Biosystems, UK). The primer sequences are provided in the table below. The standard PCR conditions were 95°C for 10 min, 40 cycles for 1 min at 94°C, annealing temperature (see table below) for 1 min and 72°C for 2 min, followed by the standard denaturation curve. The relative mRNA amount of each sample was calculated based on its threshold cycle, Ct, in comparison to the Ct of housekeeping gene β-actin. The results for cytokine or TJ mRNAs are presented as mRNA expression in epithelial cells incubated with bifidobacteria grown on HMO relative to cells incubated with LAC-grown bifidobacteria. The purity of amplified product was determined as a single peak of dissociation curve. Negative controls without RNA were also performed. Immunofluorescence assay. Epithelial cells were cultured on round glass coverslips (15mm, Fisher Scientific) in 12-well plates for 15d after reaching confluence. Cells were stimulated with HMO- or LAC-grown B. bifidum and B. infantis for 2 h. Cells were fixed in 4% paraformaldehyde for 30 minutes and non-specific background was blocked by 30 min incubation at 37°C with 20% goat serum. Slices were incubated with mouse anti-occludin 1:200 (no. 33–1500, Invitrogen, Carlsbad, CA) overnight at 4°C. The cells were then washed three times in PBS, and incubated with secondary antibody (Alexa Fluor goat anti-mouse 488 1:200; Invitrogen) for 3h at 37°C. Fluorescent images were obtained with an Olympus BX61 microscope with an Olympus DSU (spinning disk confocal) with Hamamatsu ORCA camera. MetaMorph Basic version 7.7.0.0 software was used to analyze the images. Multiple stacks (812) of sequential 0.5 ìm sections were collected for each image. From each stack, the middle sections are presented to demonstrate cytoplasmic internalization of occludin. Table S1. Primer sequences for quantitative PCR. Primer name Sequence Annealing temp Ref. β-actin forward 5’-GGCGACGAGGCCCAGAGCAAGAGAGGCAT-3’ 55°C [103] β-actin reverse 5’-CGATTTCCCGCTCGGCCGTGGTGGTGAAGC-3’ 55°C [103] IL-8 forward 5’-ATGACTTCCAAGCTGGCCGTGGCT-3’ 55°C [103] IL-8 reverse 5’-TCTCAGCCCTCTTCAAAAACTTCTC-3’ 55°C [103] IL-10 forward 5’-ATGCCCCAAGCTGAGAACCAAGACCCA-3’ 55°C [103] IL-10 reverse 5’-TCTCAAGGGGCTGGGTCAGCTATCCCA-3’ 55°C [103] TNF forward 5’-CAGAGGGAAGAGTTCCCCAG-3’ 55°C [103] TNF reverse 5’-CCTTGGTCTGGTAGGAGACG-3’ 55°C [103] Occludin forward 5′-TCAGGGAATATCCACCTATCACTTCAG-3′ 53°C [104] Occludin reverse 5′-CATCAGCAGCAGCCATGTACTCTTCAC-3′ 53°C [104] JAM-A forward 5′-TGACACGGGAAGACACTGGG-3′ 60°C [105] JAM-A reverse 5′-CTGAGCATGTCAGCACTGCC-3′ 60°C [105] ZO-1 forward 5-AGCCTGCAAAGCCAGCTCA-3’ 60°C [106] ZO-1 reverse 5-AGTGGCCTGGATGGGTTCATAG-3′ 60°C [106] SERPING forward 5’-AATGAAACCAGGGATATGATTGAC-3’ 55°C [107] SERPING reverse 5’-TTGCAAGATATGAGAAACATGGAG-3’ 55°C [107] SELPLG forward 5’-CCCAGACCACATCTCTGTGA-3’ 55°C [108] SELPLG reverse 5’-ACAGGGATGAGATGCAGACC-3’ 55°C [108] Supplemental Figure 1. Subcellular localization of occludin was assessed by immunofluorescence visualization. Epithelial cells monolayers were incubated with HMO- or LAC-grown B. bifidum and B. infantis for 2h, fixed and stained for occludin for 24h using an immunofluorescence method. Images were collected by confocal microscopy. Filed arrows indicate intracellular occludin while empty arrows represent normal intracellular spaces.