Patient Information Sheet 7
advertisement

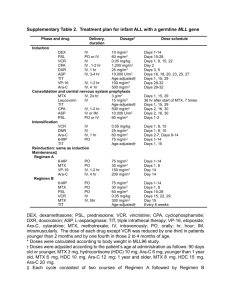
AML 17 TRIAL: PATIENT INFORMATION SHEET 7 (Ref. ISRCTN55675535) Consolidation: One versus Two Courses Of High Dose Ara-C Introduction: You have now completed 2 courses of treatment and your marrow remains in complete remission. This information sheet explains the final chemotherapy comparison we want to make in this trial. What is the purpose of this randomisation? You have had 2 courses of treatment. Your doctor considers you to be sufficiently recovered to receive the next part of your chemotherapy plan. In the past, for patients at this stage, we have tested whether another three courses had an advantage over another two courses. We found that the extra course did not improve the long term response for patients. We have some evidence that patients may do just as well with only one more course rather than two courses at this point, but we do not have enough information to be sure. So the aim of this part of the trial is to compare the effect of giving one or two more courses of chemotherapy. Patients are selected into each group in a randomised way to ensure equal spread of age and gender differences. The schedule known as high dose Ara-C which has been used for many years in standard treatment, will be used. Patients allocated to receive a second course, will receive the same treatment approximately 4 to 6 weeks later. Each course of treatment lasts for a maximum of 5 days and has very similar effects as the treatment you have already had. Patient Information and Consent form 7, 1 of 6 Version 5. May 2010 Although similar to the treatment that you already have had, these treatments usually result in your marrow taking a few days longer to recover, so that you will be in hospital for the treatment, and you will be followed closely after each course. It is likely that you will require red cell and platelet transfusion until you counts recover about four weeks after the treatment has finished, and that you will require antibiotic treatment for infection. In about 10% of patients the recovery of counts can take several weeks requiring continuous transfusion support. What will happen? If you agree you will be randomised to receive one or two further courses. However if you do not enter the two versus one randomisation you will be allocated to receive two courses because that is considered the current standard treatment. The treatment options in this randomisation are one or two courses of High Dose Ara-C You have a 50% chance of getting either treatment. Will it have side-effects? The effects of this course of treatment will be similar to those you experienced with your previous courses with some further hair loss and damage to the immune system. The pattern of the fall in your blood count, the need for transfusion support and the risk of infection requiring strong antibiotics will now be familiar to you. It is probable that your blood counts will take a few days longer to recover than they did with your previous treatments. Will this treatment benefit me? Patient Information and Consent form 7, 2 of 6 Version 5. May 2010 We do not know whether one as opposed to two more courses is beneficial until the comparison is done. You may or may not benefit more from the treatment you are allocated to. Contact for Further Information Further information can be obtained from your local organiser (Principal Investigator) or the UK organiser (Chief Investigator) whose addresses are given below. Chief Investigator: Prof Alan Burnett Department of Haematology University Hospital of Wales Cardiff CF14 4XW Tel: 029 2074 2375 e-mail: BurnettAK@cardiff.ac.uk Thank you for your participation in the trials so far and for considering this last stage of the trial. Patient Information and Consent form 7, 3 of 6 Version 5. May 2010 CONSENT FORM 7 FOR Acute Myeloid Leukaemia 17 Trial (Trial Reference ISRCTN55675535) Consolidation: One versus Two Courses Of High Dose Ara-C (Please initial) 1. I have read the attached Information Sheet version 5 dated May 2010 2. I have had an opportunity to discuss this study and ask questions 3. I have received satisfactory answers to all of my questions 4. I have received enough information about the study 5. I have spoken with Dr./ Mr./Ms.__________________________ 6. I understand that I am free to withdraw from the study: at any time without having to give reasons without affecting my future medical care Patient Information and Consent form 7, 4 of 6 Version 5. May 2010 7. I understand that sections of my medical records relating to my participation in the study may be inspected by responsible individuals from the trial Sponsor who is Cardiff University. All personal details will be treated as STRICTLY CONFIDENTIAL. The information will be used for medical research only and I will be identified only by trial number, initials and date of birth. I will not be identified in any way in analysis and reporting of the results. I give permission for these individuals to have access to my records and to have my clinical details recorded in this way 8. I agree to participate in this study 9. I give permission to tell my GP about my participation in the study Patient’s Signature:_____________________________________ Name in block letters:___________________________________ Date_____________________ Doctor’s Signature:_____________________________________ Name in block letters:___________________________________ Date_____________________ Patient Representative’s Signature: (if appropriate)_________________________________________ Name in block letters:___________________________________ Relationship to patient: __________________________________ Patient Information and Consent form 7, 5 of 6 Version 5. May 2010 Date_____________________ Patient Information and Consent form 7, 6 of 6 Version 5. May 2010