PRO_2003_sm_suppinfo
advertisement

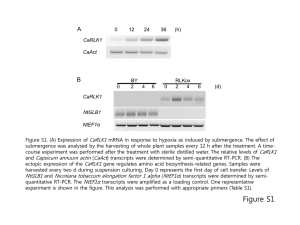
Structural basis of the human AM-receptor Supplementary Materials MATERIALS AND METHODS Calibration of molecular mass The molecular masses of the purified RAMP2 and the CRLR•RAMP2 complex were analyzed by size-exclusion chromatography. The samples were applied to a Superdex 200 HR 10/30 (GE Healthcare Bio-Sciences) column and eluted with 20 mM Tris-HCl buffer (pH 8.0), containing 150 mM NaCl. The flow rate was 0.5 ml/ml, and the UV absorbance at 280 nm was monitored. Molecular mass calibration was performed using bovine albumin (66 kDa), egg albumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and -lactalbumin (14.4 kDa) as standards. Protein photo-cross-linking in mammalian cells The CRLR gene, C-terminally tagged with a c-myc epitope, was cloned in the pcDNA4/TO vector (Invitrogen). The RAMP2 gene, with a TEV-cleavable site at the C-terminus followed by a FLAG sequence, was cloned in the pOriP vector 1. The RAMP2 gene variants with amber codons at positions 93, 94, 97, 117, 120, 121, 124, and 128 were created using a QuikChange site-directed mutagenesis kit (Agilent Technologies). The gene encoding a pBpa-specific variant of E. coli TyrRS or Eco-pBpaRS, together with nine copies of the gene encoding the Bacillus stearothermophilus amber suppressor tRNA 2, was cloned in pOriP, to create pSuPT-pBpaRS. The HEK 293 c-18 cells (ATCC) were transfected with these expression plasmids, using the Lipofectamine 2000 reagent (Invitrogen) in Opti-MEMI growth medium (Invitrogen), as described previously 2. Four 1 Structural basis of the human AM-receptor hours after transfection, the growth medium was replaced by serum-free D-MEM medium (Invitrogen) containing 0.5 mM pBpa, and the cells were further incubated at 37 ºC for 16 hours. Photo-cross-linking between CRLR and RAMP variants with pBpa was performed as described 3. Cell extracts were prepared with lysis buffer [30 mM Tris-HCl (pH 7.4), 10% glycerol, 150 mM NaCl, 5 mM EDTA, 1.5% n-dodecyl-maltoside (DDM), and protease inhibitor cocktail at a 1:100 dilution (Nacalai Tesque, Kyoto, Japan)], and the cross-linked products were purified by a one-hour incubation at 4 ºC with anti-FLAG M2 affinity gel (Sigma). The gel was washed once with lysis buffer, and twice with wash buffer [30 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 0.05 % DDM], and the products were then eluted by FLAG peptides (Sigma). The eluates were fractionated on 4-12 % Nu-PAGE gradient gels (Invitrogen), transferred to PVDF membranes (Millipore), and detected by western blotting with an anti-FLAG antibody (Sigma) or an anti-myc antibody (Cell Signaling Technology). AM- and CGRP-binding assays The 52-amino acid peptide of human AM (YRQSMNNFQGLRSFGCRFGTCTVQKLAHQIYQFTDKDKDNVAPRSKISPQGY) was synthesized at the Support Unit for Bio-material Analysis in the RIKEN BSI Research Resources Center (RRC), and CGRP (1-37) was purchased from SIGMA. Point mutations were introduced into the CRLR and RAMP2 ECDs by using a QuikChange site-directed mutagenesis kit (Agilent Technologies). The complex between the ECDs of human CRLR (residues 23–136) and RAMP1 (residues 27–112) (the CRLR•RAMP1 complex) was prepared by the same method as described for the CRLR•RAMP2 complex. The in vitro binding assay was performed by SPR measurements, using a Biacore instrument (Biacore 2 Structural basis of the human AM-receptor 3000; GE Healthcare). Proteins were diluted in HBS-EP running buffer, which contained 10 mM HEPES, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, and 0.005 % Tween 20. In a series of experiments, CRLR, the CRLR•RAMP2 complex, and the CRLR•RAMP1 complex were immobilized onto a CM5 sensor chip (GE Healthcare), using amine-coupling chemistry, leading to 1,000 RU. As a control, bovine serum albumin was immobilized onto the flow cells of a CM5 sensor chip, leading to 1,000 RU. The experiments were performed at 25 °C, and at a flow rate of 20 l/min. For each experiment, at least five different concentrations of ligands (AM: 1.0, 0.5, 0.25, 0.125, and 0.0625 M; CGRP: 10, 5, 2.5, 1.25, and 0.625 M) were injected for 2 min, and dissociative reactions were monitored for 10 min. The KD values were obtained from the steady-state curve fitting analysis, using the BIA evaluation software (GE Healthcare). REFERENCES 1. Mukai, T., Kobayashi, T., Hino, N., Yanagisawa, T., Sakamoto, K. & Yokoyama, S. (2008). Adding l-lysine derivatives to the genetic code of mammalian cells with engineered pyrrolysyl-tRNA synthetases. Biochem Biophys Res Commun 371, 818-22. 2. Hino, N., Hayashi, A., Sakamoto, K. & Yokoyama, S. (2006). Site-specific incorporation of non-natural amino acids into proteins in mammalian cells with an expanded genetic code. Nat Protoc 1, 2957-62. 3. Hino, N., Okazaki, Y., Kobayashi, T., Hayashi, A., Sakamoto, K. & Yokoyama, S. (2005). Protein photo-cross-linking in mammalian cells by site-specific incorporation of a photoreactive amino acid. Nat Methods 2, 201-6. 3 Structural basis of the human AM-receptor FIGURE LEGENDS FIGURE S1. Size-exclusion chromatography of the CRLR•RAMP2 complex. (A) Elution profile of the purified CRLR•RAMP2 complex on a Superdex 200 HR10/30 gel-filtration column, monitored by the absorbance at 280 nm. (B) Elution profile of the purified CRLR under the same conditions as in (A). (C) Calibration curve based on the elution profiles of five standards, indicating that the molecular masses of the CRLR•RAMP2 complex at protein concentrations of 0.5, 1.0, 5.0 mg/ml are about 26 kDa, 29 kDa, and 40 kDa, respectively, which are close to the theoretically calculated masses of one and two CRLR•RAMP2 heterodimers (24.3 kDa and 48.6 kDa, respectively). The molecular mass of CRLR is about 16 kDa, which is close to the theoretically calculated mass (13.8 kDa). (D), (E) Purified CRLR•RAMP2 complex and CRLR, analyzed by SDS-PAGE. Proteins were visualized by Coomassie Brilliant Blue staining. The positions of the molecular mass markers (in kDa) are shown on the left. FIGURE S2. Crystal packing interactions of the CRLR•RAMP2 complex. (A) Structure of the CRLR•RAMP2 complex (the B•A molecules), represented with the symmetry-related complex (the C•D molecules). (B) Molecular surface representation of a pair of symmetry-related CRLR•RAMP2 heterodimer complexes. The top and bottom views are related by a 180° rotation about the vertical axis. For each protein, the glycosylation sites are colored green. (C) Hydrogen bond interactions between molecule A and molecule C, and between molecule B and molecule D. In the close-up views of the interfaces, the interacting residues are displayed as stick models. Hydrogen bonds are indicated as dashed lines. The coloring scheme is the same as in Figure 1B. (D) The 4 Structural basis of the human AM-receptor hydrophobic interactions and the hydrogen bonds are represented between molecule B (CRLR) and molecule D (RAMP2). The two views are related by a 90° rotation about the horizontal axis. FIGURE S3. Sequence analysis and surface properties of CRLR. (A) Cartoon presentation and electrostatic surface of CRLR at the CRLR•RAMP2 interface. Blue and red surfaces represent positive and negative potentials, respectively. The circle indicates the location of the hydrophobic patch, which forms an interactive face with RAMP2. (B) Sequence alignment of human CRLR and other class B GPCRs (hCTR, hPTH1R, hCRFR1, hGIPR, hGLP1R, hPAC1R), excluding the signal peptide sequences and the transmembrane regions. The sequence analysis was generated and represented as in Figure 2A. The secondary structure of CRLR is shown above the sequences. The numbers below the sequences indicate the positions of the cysteine residues involved in the formation of the first, second, and third disulfide bonds (Cys48–Cys74, Cys65–Cys105, and Cys88–Cys127, respectively). (C) Superimposition of the C atoms of PTH•PTH1R (PDB ID: 3C4M), GIP•GIPR (2QKH), GLP•GLP1R (3C5T), CRF•CRFR1 (3EHU), and PACAP•PAC1R (2JOD) onto those of the CRLR extracellular domain in the CRLR•RAMP2 complex. 5 Structural basis of the human AM-receptor TABLE S1. X–ray data collection, phasing and refinement statistics of RAMP2 RAMP2_ Peak Data collection Wavelength (Å) RAMP2_ Edge RAMP2_ Remote 0.9788 0.9792 0.9640 50–2.0 (2.07–2.00) 33465 50–2.0 (2.07–2.00) 33672 50–2.0 (2.07–2.00) 33794 7.00 6.54 6.48 Completeness (%) 100 (99.8) 100 (98.7) 100 (98.9) I / (I) 20.4 (9.21) 20.9 (7.72) 20.3 (6.53) Rsym1 8.5 (23.1) 7.6 (26.0) 7.9 (30.5) Resolution (Å) Unique reflections Redundancy (%) MAD analysis Resolution (Å) 2.0 No. of sites 12 FOMMAD 2 FOMRESOLVE 0.46 3 Refinement Resolution (Å) 0.62 36.4–2.0 No. of reflections 31769 No. of protein atoms 3815 No. of water molecules 282 Rwork (%) 23.0 4 27.6 Rfree (%) r.m.s.d. bond length (Å) r.m.s.d. bond angles (˚) 0.003 1.1 Ramachandran plot Most favored regions (%) 100 Additional allowed regions (%) 0.0 Generously allowed regions (%) 0.0 Disallowed regions (%) 0.0 All numbers in parentheses represent last outer shell statistics. 1R sym= |Iavg–Ii| / Ii, where Ii is the observed intensity and Iavg is the average intensity. 2Figure of merit after SOLVE phasing. 3Figure of merit after RESOLVE. 4R free is calculated for 10 % of randomly selected reflections excluded from refinement. 6