Radiolabeled Primer Cycle Sequencing and
advertisement
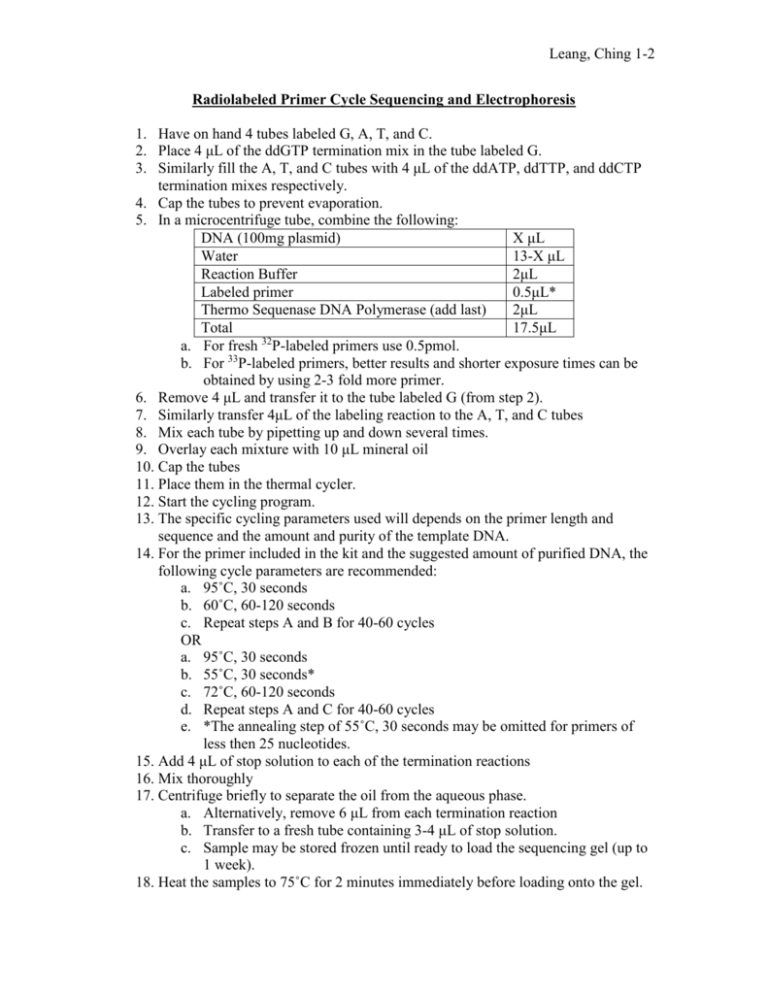
Leang, Ching 1-2 Radiolabeled Primer Cycle Sequencing and Electrophoresis 1. Have on hand 4 tubes labeled G, A, T, and C. 2. Place 4 μL of the ddGTP termination mix in the tube labeled G. 3. Similarly fill the A, T, and C tubes with 4 μL of the ddATP, ddTTP, and ddCTP termination mixes respectively. 4. Cap the tubes to prevent evaporation. 5. In a microcentrifuge tube, combine the following: DNA (100mg plasmid) X μL Water 13-X μL Reaction Buffer 2μL Labeled primer 0.5μL* Thermo Sequenase DNA Polymerase (add last) 2μL Total 17.5μL a. For fresh 32P-labeled primers use 0.5pmol. b. For 33P-labeled primers, better results and shorter exposure times can be obtained by using 2-3 fold more primer. 6. Remove 4 μL and transfer it to the tube labeled G (from step 2). 7. Similarly transfer 4μL of the labeling reaction to the A, T, and C tubes 8. Mix each tube by pipetting up and down several times. 9. Overlay each mixture with 10 μL mineral oil 10. Cap the tubes 11. Place them in the thermal cycler. 12. Start the cycling program. 13. The specific cycling parameters used will depends on the primer length and sequence and the amount and purity of the template DNA. 14. For the primer included in the kit and the suggested amount of purified DNA, the following cycle parameters are recommended: a. 95˚C, 30 seconds b. 60˚C, 60-120 seconds c. Repeat steps A and B for 40-60 cycles OR a. 95˚C, 30 seconds b. 55˚C, 30 seconds* c. 72˚C, 60-120 seconds d. Repeat steps A and C for 40-60 cycles e. *The annealing step of 55˚C, 30 seconds may be omitted for primers of less then 25 nucleotides. 15. Add 4 μL of stop solution to each of the termination reactions 16. Mix thoroughly 17. Centrifuge briefly to separate the oil from the aqueous phase. a. Alternatively, remove 6 μL from each termination reaction b. Transfer to a fresh tube containing 3-4 μL of stop solution. c. Sample may be stored frozen until ready to load the sequencing gel (up to 1 week). 18. Heat the samples to 75˚C for 2 minutes immediately before loading onto the gel. Leang, Ching 2-2 a. Heating in open vials will promote evaporation of water from the formamide-reaction mixture. b. This will increase the signal by concentrating the isotope and promote more complete denaturation of the DNA. c. Avoid overheating. 19. Load 2-3 μL in each gel lane. General guidelines for electrophoresis: 1. Ultrapure or electrophoresis grade reagents should be used. 2. Store solutions no longer than one week in the dark at 4˚C. 3. Commercial preparations on acrylamide gel mixes in liquid or powder form should be used according to manufactures recommendations. 4. Gels should be prepared 2-20 hours prior to use, and pre-run for ~15 minutes. 5. The use of tapered spaces (‘wedge’ gels) improves overall resolution and allows more nucleotides to be read from a single loading. 6. Load 8 adjacent lanes in the pattern GATCGTAC, which aids reading closely spaced bands. 7. Gel runs of 18-24 hour at 40-50 watts are often necessary for reading in the 400600bp range. a. When reading longer sequences, it is usually convenient to run gels overnight with a time-controlled power supply. 8. Gels should be soaked in 5% acetic acid, 15% methanol to remove the urea. a. Soaking time depends on gel thickness. b. Approximate minimum times are: i. 5 minutes for 0.2mm gels ii. 15 minutes for 0.4mm gels iii. 60 minutes for field gradient (0.4-1.2mm wedge) or formamide gels. 9. Drying should be done at moderate temperature (80˚C) to preserve resolution. 10. If RapidGel-XL is used, the gel does not need to be soaked. a. In fact, soaking RapidGel-XL gels will cause swelling thereby affecting band resolution in the final result. b. Gels dried without prior soaking will require longer drying and exposure times but give sufficient resolution for most purposes. 11. Leaving plastic-wrap on helps to prevent the film from sticking to incompletelydried gels 12. For 35S or 33P gels, exposure must be done with direct contact between the dried gel and the emulsion side of the film. 13. In general, overnight to 35 hour exposures are sufficient when using fast film such as HyperfilmTM-MP. a. Use Kodak BiomaxTM MR or HyperfilmTM-βmax autoradiography film.











