seasonal hydrological effects on secondary production of
advertisement

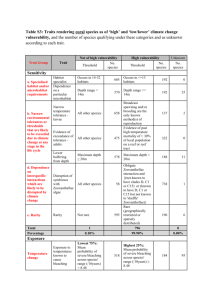
SEASONAL HYDROLOGICAL EFFECTS ON SECONDARY PRODUCTION OF HYDROPSYCHIDAE IN REACH 6, UPPER MISSISSIPPI RIVER Introduction Seasonal changes in a river are a way of life for organisms and can affect them in many different ways. Thus, their life cycle develops a pattern with these changes. Larger rivers have a smoother seasonal flood pattern than smaller rivers due to their larger watershed areas that can absorb more water into their system than smaller rivers and streams. Floodplain areas have different production rates due to the seasonal fluctuation rates and can affect production at greater rates than other areas (Smock et al. 1992). This is due to nutrients that are brought into use when floodplains are immersed with water. Temperate habitats generally result in specific growth rates of organisms and the production of populations that vary along with season and follow the temperature cycle (Banse and Mosher 1980). Because of this, invertebrate production often differs between habitats. Community composition and secondary production will differ among sites within a large river in response to both physical characteristics of each habitat and the types of organic matter present (Thorp and Delong 1994). Increased velocity, substrate instability, and scouring of substrate surface are commonly associated with floods and can harm benthic organisms. However, species composition and relative abundance of many benthic communities remain relatively constant through time and over multiple floods (Rempel et al. 1999). Modes of action of the hydrologic disturbances on stream communities may work by erosion when habitats are scoured by floods, or by desiccation when aquatic habitats decrease gradually and organisms become stranded above the water surface (Feminella and Resh 1990). Flow refugia reduce the loss of organisms during flooding and allow benthic populations to thrive (Rempel et al. 1999). Caddisfly larvae contribute significantly to zoobenthic biomass and the processing of organic resources. Larvae, which are particularly responsive to instream environmental gradients, are restricted to the permanently wetted stream perimeter. Benthic habitat is reduced through alterations of natural-flow conditions during spring and summer, but enhanced during fall and winter (Hauer and Stanford 1991). Georgian and Thorp (1992) noted that large larvae feed selectively on high-quality prey food items such as diatoms and drifting invertebrates. When there is an increase in density of drifting invertebrates (Benke and Wallace 1997), increased food quality results (Benke and Wallace 1997). Hydropsychidae caddiflies, the focus of this study, are ideal organisms for life history and production analyses. They generally have five distinct instars, which take 2-3 mon. to go from egg to adult emergence (Benke et al. 1984). Cohort production is the accumulation of flows over that population-specific interval (Benke 1993). When an aquatic animal population grows as a synchronous cohort in nature, mortality and individual growth are easily incorporated into an estimate of secondary production using a cohort technique (Benke et al. 1998). Floodplain river instream primary production is a significant contribution to secondary productivity (Thorp and Delong 1994). Secondary production is the most comprehensive measure of success for a population because it is a composite of several other components of success: density, biomass, individual growth rate, reproduction, survivorship, and development time. Since production is directly related to consumption, it represents a quantification of a population’s resource use in a given time interval (Benke and Jacobi 1994). Thus, secondary production is a response variable that deals with the formation of heterotrophic biomass through time. These measurements allow one to determine the absolute and relative amounts of food resources consumed (Benke 1993). Also, production analysis provides a common link between populations and ecosystems as it represents a measure of a population’s function at the community and ecosystem level. Populations, functional feeding groups, and consumers as a whole are dynamic components of ecosystems and production is the ultimate dynamic variable. Biomass is simply how much living tissue mass is present at a given time and production is a flow of mass or energy, as observed by Benke (1993). The objective of this study was to determine if river hydrology fluctuations affect secondary production of Hydropsychidae in the Upper Mississippi River. Methods Samples were collected from two sites on the Upper Mississippi River in Reach 6, near Winona, MN. Rock samples were taken from wing dams at each site. Site 1 was located on the right bank near first light below dam 5A at river mile 727.9. Site 2 was also on the right bank at river mile 726.3. Both sites are representative of the upper end of a navigation reach, with hydrological patterns expected to follow near-historical patterns (Sparks 1995). Rock samples were collected approximately every 3 wks. Sample dates were June 30, July 21, August 11, August 29, September 20, and October 11, 2000 (Figure 1). A minimum of ten samples were collected on each sample date by removing five rocks from each site on every sample date. Measurements of pH, depth, turbidity, and current velocity were recorded for each sample to monitor any changes over the collecting period that might affect our sampling of caddisfly larvae. Upon collection, rock samples were placed in plastic bags with labels and filled with 70% ethanol. Rocks were scrubbed in the laboratory initially by hand, then with a brush to remove all invertebrates from the substrate. This material was rinsed through a 0.1-mm sieve and stored in a container with 70% ethanol. Rocks were measured by length, height, and width for determination of surface area. Caddisfly larvae were sorted, counted, and placed in vials, with ethanol, according to size (large or small) and species. Larvae were split into two groups, small and large, because previous studies in the Large River Studies Center had indicated two cohorts of Hydropsychidae exist in the Upper Mississippi River. Larval biomass was determined by using length-mass relationships, using equations that predict mass as a power function of a linear dimension, particularly body length and head width: M=aLb, where M is larvae mass (mg), L is any linear dimension (mm), and a and b are constants as outlined by Benke et al. (1999). Cohort production was calculated for the intervals between sampling dates: June 30-July 21, July 21-August 11, August 11-August29, August 29-September 20, and September 20-October 11 (Appendix 1). Production was calculated using the increment summation method using the equation P=N W, where P is production over a time interval, N is the mean density of larvae, and W is the difference between final and initial mean larval weight (Butler 1982). Results Discharge fluctuated throughout the study period (Figure 1). The spring 2000 flood did not match the historical average. Hydrological patterns were not at typical times of the year. Mid-June through July showed a discharge higher than normal. After this high discharge period, rates of production and density were, for the most part, at their highest rates. Water temperatures typically fluctuated between 200 and 260C during the summer months and went down to 100C for the last sample date in October. Three species of Hydropsychidae were identified: Cheumatopsyche sp., Hydropsyche orris, and Potamyia flava. While two cohorts had been identified, overlap during August-September made it difficult to separate them. Therefore, the focus became changes in production of the size groups for each species. As shown in Figure 2, average densities of large Cheumatopsyche sp. larvae were greatest at the beginning of August and lowest during rest of months. Densities of large H. orris were lowest in June, then increased July through August before decreasing in September-October. Large P. flava densities were lowest in June and increased to about the same level for the rest of the year. Figure 3 shows that the densities of small Cheumatopsyche sp. larvae were highest during July-August and lowest in June. Small H. orris larvae densities were highest in July and about the same during the other months except in September, when no small larvae were found. Small P. flava larvae, which had lowest overall densities, followed a pattern similar to Cheumatopsyche sp. larvae. Production patterns were the greatest for both large and small H. orris larvae during July-August interval (Figure 4). H. orris production at first increased until it reached its climax then dropped off dramatically by the middle of August. No production estimates were found during the August-September interval. Large P. flava larvae production was highest in July-August and lowest throughout rest of the study period (Figure 5). Small larvae production generally stayed low except for a slight increase in September-October. Production for small Cheumatopsyche sp. larvae were lowest in June, while reaching a maximum in July-August, before dropping in late August and increasing in September (Figure 6). Large Cheumatopsyche sp. larvae production increased from June and reached the highest rate in August, before dropping in September and then again increasing by September-October. Discussion Hydropsychidae are known to utilize microhabitats with large stable substrate and high water flow velocity. Current velocity and flow patterns would be expected to have a direct influence on these filter feeders. Highest abundances are known to have occurred on the upstream and top position of substrate, where the highest flows occur (Georgian and Thorp 1992). Larval abundance can vary with depth, often as a function of oxygen concentrations as affected substrate type and interstitial flow variability (Smock et al. 1992). Hydrological events can reduce the density of caddisfly larvae occupying substrate in a particular area. Increases and decreases of densities can be partially attributed to this; however, densities of small caddisfly larvae increased July through August due to the hatching of new eggs. Decreases after August are probably due to the size change when small larvae were then categorized as large, thus accounting for the dramatic increase in numbers of large larvae in August. Decrease of large larvae densities at the end of August is due to emergence. This typically takes place over late July, August, and September (Beckett 1982). Secondary production of larvae increases July through August, generally for all three species and both sizes. It is even more pronounced in large larvae production. This is due to emergence preparation of large larvae. Declines in small larvae are due to mortality as larvae and losses during aestivation and pupation (Whiles et al. 1999). While in large larvae, loss is due greatly to preparation for emergence and actual emergence of adults. Differences in substrate stability affect productivity. A positive relationship exists between productivity and water flow. A high level of secondary production requires a nearby center of primary production, which is during the summer/fall low-flow period, rather than long-distance transport of organic matter from upstream sources via the main channel (Thorp and Delong 1994). Thus, production is the lowest during periods of high discharge in the spring and greatest during periods of low discharge in late summer. At times when there is more food in the water and less disturbance, production can increase to its maximum. Increases in secondary production in this study did occur when river discharge rose. Overall, production was lowest during high discharge (June-July) and highest during receding and low-flow conditions (JulyAugust). Literature Cited Banse, K. and S. Mosher. 1980. Adult body mass and annual production/biomass relationships of field populations. Ecological Monographs 50:355-379. Beckett, D.C. 1982. Phenology of Hydropsyche orris (Trichoptera: Hydropsychidae) in the Ohio River: Changes in larval age structure and substrate colonization rates. Environmental Entomology 11:1154-1158. Benke, A.C. 1993. Concepts and patterns of invertebrate production in running waters. Verh. Internat. Verein. Limnol. 25:15-38. Benke, A.C., A.D. Huryn, and G.M. Ward. 1998. Use of empirical models of stream invertebrate secondary production as applies to a functional feeding group. Verh. Internat. Verein. Limnol. 26:2024-2029. Benke, A.C. and D.I. Jacobi. 1994. Production dynamics and resource utilization of snag-dwelling mayflies in a blackwater river. Ecology 75:1219-1232. Benke, A.C. and J.B. Wallace. 1997. Trophic basis of production among riverine caddisflies: implications for food web analysis. Ecology 78:1132-1145. Benke, A.C., T.C. Van Arsdall Jr., D.M. Gillespie, and F.K. Parrish. 1984. Invertebrate productivity in a subtropical blackwater river: the importance of habitat and life history. Ecological Monographs 54:25-63. Benke, A.C., A.D. Huryn, L.A. Smock, and J.B. Wallace. 1999. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the Southeastern United States. Journal of the North American Benthological Society. 18:308-343. Butler, M.G. 1982. Production dynamic of some artic Chironomus larvae. American Society of Limnology and Oceanography 27:728-736. Feminella, J.W. and V.H. Resh. 1990. Hydrologic influences, disturbance, and intraspecific competition in a stream caddisfly population. Ecology 71:20832094. Georgian, T. and J.H. Thorp. 1992. Effects of microhabitat selection on feeding rates of net-spinning caddisfly larvae. Ecology 73:229-240. Hauer, F.R. and J.A. Stanford. 1991. Distribution and abundance of Trichoptera in a large regulated river. Verh. Internat. Verein. Limnol. 24:1636-1639. Rempel, L.L., J.S. Richardson, and M.C. Healey. 1999. Flow refugia for benthic macroinvertebrates during flooding of a large river. Journal of the North American Benthological Society 18:34-48. Smock, L.A., J.E. Gladden, J.L. Riekenberg, L.C. Smith, and C.R. Black. 1992. Lotic macroinvertebrate production in three dimensions: channel surface, hyporheic, and floodplain environments. Ecology 73:876-886. Thorp, J.H. and M.D. Delong. 1994. The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems. Forum 70: 305-308. Whiles, M.R., B.S. Goldowitz, and R.E. Charlton. 1999. Life history and production of a semi-terrestrial limnephilid caddisfly in an intermittent Platte River wetland. Journal of the North American Benthological Society 18:533-544. Figure Headings Figure 1: Average historical daily discharge (cfs) compared to 2000-year daily discharge of Upper Mississippi River, near Winona, MN (* denotes sample dates). Figure 2: Densities (number/m2) of large Cheumatopsyche sp., Hydropsyche orris, and Potamyia flava, from Reach 6. Bar denotes + 1 SE. Figure 3: Densities (number/m2) of small Cheumatopsyche sp., Hydropsyche orris, and Potamyia flava, from Reach 6, Upper Mississippi River on each sampling date, near Winona, MN, 2000. Rocks collected from wing dams. Bar denotes + 1 SE. Figure 4: Secondary production (mg/m2/d) of large and small Hydropsyche orris. Figure 5: Secondary production (mg/m2/d) of large and small Potamyia flava from Reach 6, Upper Mississippi River, near Winona, MN, 2000. Sampling dates were from June through October 2000. Figure 6: Secondary production (mg/m2/d) of large and small Cheumatopsyche sp. from Reach 6, Upper Mississippi River, near Winona, MN, 2000. Sampling dates were from June through October 2000.